|
Fispemifene
Fispemifene (INN, USAN) (developmental code name HM-101) is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group that was developed for the treatment of male hypogonadism but was abandoned and never marketed. It reached phase II clinical trials for this indication before development was terminated in March 2016. The drug failed to achieve statistical significance on key effectiveness endpoint An endpoint, end-point or end point may refer to: * Endpoint (band), a hardcore punk band from Louisville, Kentucky * Endpoint (chemistry), the conclusion of a chemical reaction, particularly for titration * Outcome measure, a measure used as an e ...s in clinical trials and was discontinued by its developer for strategic reasons. See also * Ospemifene References External links Fispemifene - AdisInsight Primary alcohols Organochlorides Progonadotropins Selective estrogen receptor modulators Triphenylethylenes {{genito-urinary-drug-s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
United States Adopted Name
A United States Adopted Name (USAN) is a unique nonproprietary name assigned to a medication marketed in the United States. Each name is assigned by the USAN Council, which is co-sponsored by the American Medical Association (AMA), the United States Pharmacopeial Convention (USP), and the American Pharmacists Association (APhA). The USAN Program states that its goal is to select simple, informative, and unique nonproprietary names (also called generic names) for drugs by establishing logical nomenclature classifications based on pharmacological or chemical relationships. In addition to drugs, the USAN Council names agents for gene therapy and cell therapy, List of soft contact lens materials, contact lens polymers, surgical materials, diagnostics, carriers, and substances used as an excipient. The USAN Council works in conjunction with the World Health Organization (WHO) international nonproprietary name (INN) Expert Committee and national nomenclature groups to standardize drug ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nonsteroidal
A nonsteroidal compound is a drug that is not a steroid nor a steroid derivative. Nonsteroidal anti-inflammatory drugs (NSAIDs) are distinguished from corticosteroids as a class of anti-inflammatory agents. List of nonsteroidal steroid receptor modulators Examples include the following: * Estrogens: benzestrol, bifluranol, estrobin (DBE), diethylstilbestrol (stilbestrol), dienestrol, erteberel, fosfestrol, hexestrol (dihydroxystilbestrol), methallenestril, methestrol, methestrol dipropionate, paroxypropione, prinaberel, and triphenylethylene, as well as many xenoestrogens * : acolbifene, afimoxifene, arzoxifene, bazedoxifene, broparestrol, chlorotrianisene, clomifene, clomifenoxide, cyclofenil, droloxifene, enclomifene, endoxifen, ethamoxytriphetol, fispemifene, idoxifene, lasofoxifene, levormeloxifene, miproxifene, nafoxidine, nitromifene, ormeloxifene, ospemifene, panomifene, pipendoxifene, raloxifene, tamoxifen, toremifene, trioxifene, zindoxifene, zuclomifene * An ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selective Estrogen Receptor Modulator
Selective estrogen receptor modulators (SERMs), also known as estrogen receptor agonist/antagonists (ERAAs), are a class of drugs that act on the estrogen receptor (ER). A characteristic that distinguishes these substances from pure ER agonists and antagonists (that is, full agonists and silent antagonists) is that their action is different in various tissues, thereby granting the possibility to selectively inhibit or stimulate estrogen-like action in various tissues. Medical uses SERMs are used for various estrogen-related diseases, including treatment of ovulatory dysfunction in the management of infertility, treatment and prevention of postmenopausal osteoporosis, treatment and reduction in risk of breast cancer and treatment of dyspareunia due to menopause. SERM is also used in combination with conjugated estrogens indicated for the treatment of estrogen deficiency symptoms, and vasomotor symptoms associated with menopause. SERMs are used dependent on their pattern of acti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylethylene
Triphenylethylene (TPE) is a simple aromatic hydrocarbon that possesses weak estrogenic activity. Its estrogenic effects were discovered in 1937. TPE was derived from structural modification of the more potent estrogen diethylstilbestrol, which is a member of the stilbestrol group of nonsteroidal estrogens. TPE is the parent compound of a group of nonsteroidal estrogen receptor ligands. It includes the estrogens chlorotrianisene, desmethylchlorotrianisene, estrobin (DBE), M2613, triphenylbromoethylene, triphenylchloroethylene, triphenyliodoethylene, triphenylmethylethylene; the selective estrogen receptor modulators (SERMs) afimoxifene, brilanestrant, broparestrol, clomifene, clomifenoxide, droloxifene, endoxifen, etacstil, fispemifene, idoxifene, miproxifene, miproxifene phosphate, nafoxidine, ospemifene, panomifene, and toremifene. The antiestrogen ethamoxytriphetol (MER-25) is also closely related, but is technically not a derivative of TPE and is instead a triphenyleth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypogonadism
Hypogonadism means diminished functional activity of the gonads—the testes or the ovaries—that may result in diminished production of sex hormones. Low androgen (e.g., testosterone) levels are referred to as hypoandrogenism and low estrogen (e.g., estradiol) as hypoestrogenism. These are responsible for the observed signs and symptoms in both males and females. Hypogonadism, commonly referred to by the symptom "low testosterone" or "Low T", can also decrease other hormones secreted by the gonads including progesterone, DHEA, anti-Müllerian hormone, activin, and inhibin. Sperm development (spermatogenesis) and release of the egg from the ovaries (ovulation) may be impaired by hypogonadism, which, depending on the degree of severity, may result in partial or complete infertility. In January 2020, the American College of Physicians issued clinical guidelines for testosterone treatment in adult men with age-related low levels of testosterone. The guidelines are supported b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phases Of Clinical Research
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for safety in a few human subjects, then expand to many study participants (potentially tens of thousands) to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays. Summary Clinical trials testing potential medical products are commonly classified into four phases. The drug development process will normally proceed through all four phases over many years. If the drug successfully passes through Phases I, II, and III, it will usually be approved by the national regulatory authority for use in the general population. Phase IV trials are 'post-marketing' or 'surveillance' studies conducted to monitor safety over sever ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Trial
Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted. Depending on product type and development stage, investigators initially enroll volunteers or patients into small pilot studies, and subsequently conduct progressively larger scale comparative studies. Clinical trials can vary i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
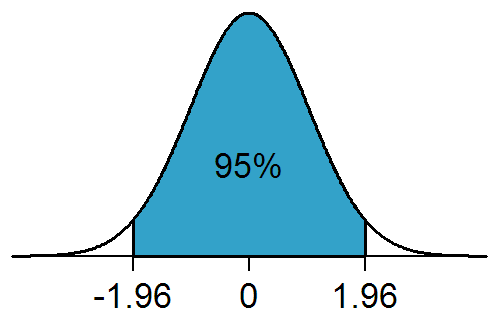
Statistical Significance
In statistical hypothesis testing, a result has statistical significance when it is very unlikely to have occurred given the null hypothesis (simply by chance alone). More precisely, a study's defined significance level, denoted by \alpha, is the probability of the study rejecting the null hypothesis, given that the null hypothesis is true; and the ''p''-value of a result, ''p'', is the probability of obtaining a result at least as extreme, given that the null hypothesis is true. The result is statistically significant, by the standards of the study, when p \le \alpha. The significance level for a study is chosen before data collection, and is typically set to 5% or much lower—depending on the field of study. In any experiment or observation that involves drawing a sample from a population, there is always the possibility that an observed effect would have occurred due to sampling error alone. But if the ''p''-value of an observed effect is less than (or equal to) the significanc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Effectiveness
Effectiveness is the capability of producing a desired result or the ability to produce desired output. When something is deemed effective, it means it has an intended or expected outcome, or produces a deep, vivid impression. Etymology The origin of the word "effective" stems from the Latin word effectīvus, which means creative, productive or effective. It surfaced in Middle English between 1300 and 1400 A.D. Usage In mathematics and logic, ''effective'' is used to describe metalogical methods that fit the criteria of an effective procedure. In group theory, a group element acts ''effectively'' (or ''faithfully'') on a point, if that point is not fixed by the action. In physics, an effective theory is, similar to a phenomenological theory, a framework intended to explain certain (observed) effects without the claim that the theory correctly models the underlying (unobserved) processes. In heat transfer, ''effectiveness'' is a measure of the performance of a heat exchange ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Endpoint
Clinical endpoints or clinical outcomes are Outcome measure, outcome measures referring to occurrence of disease, symptom, Medical sign, sign or laboratory abnormality constituting a target outcome in clinical trial, clinical research trials. The term may also refer to any disease or sign that strongly motivates withdrawal of an individual or entity from the trial, then often termed a ''humane (clinical) endpoint''. The primary endpoint of a clinical trial is the endpoint for which the trial is statistical power, powered. Secondary endpoints are additional endpoints, preferably also pre-specified, for which the trial may not be powered. Surrogate endpoint, Surrogate endpoints are trial endpoints that have outcomes that substitute for a clinical endpoint, often because studying the clinical endpoint is difficult, for example using an increase in blood pressure as a surrogate for death by cardiovascular disease, where strong evidence of a causal link exists. Scope In a general sens ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ospemifene
Ospemifene (brand names Osphena and Senshio produced by Shionogi) is an oral medication indicated for the treatment of dyspareuniapain during sexual intercourseencountered by some women, more often in those who are post-menopausal. Ospemifene is a selective estrogen receptor modulator (SERM) acting similarly to an estrogen on the vaginal epithelium, building vaginal wall thickness which in turn reduces the pain associated with dyspareunia. Dyspareunia is most commonly caused by "vulvar and vaginal atrophy." The medication was approved by the FDA in February 2013 and by the European Commission for marketing in the EU in January 2015. Medical uses Ospemifene is used to treat dyspareunia. In the US it is indicated for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy (VVA), due to menopause. In the EU it is indicated for the treatment of moderate to severe symptomatic VVA in post-menopausal women who are not candidates for local vaginal oest ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)