|
Fire Classes
A fire class is a system of categorizing fire with regard to the type of material and fuel for combustion. Class letters are often assigned to the different types of fire, but these differ between territories. There are separate standards for the United States, Europe, and Australia. This is used to determine the type of extinguishing agent that can be used for that fire class. Class A: Ordinary combustibles Class A fires consist of ordinary combustibles such as wood, paper, fabric, and most kinds of trash. They may be extinguished by water, wet chemical suppression, or dry chemical powder. Class B: Flammable liquid Class B fires are those where the fuel is flammable or combustible liquid. The US system includes flammable gases in their "Class B". In the European/Australian system, flammable liquids are designated "Class B" having flash point less than . These fires follow the same basic fire tetrahedron (heat, fuel, oxygen, chemical reaction) as ordinary combustible ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fire
Fire is the rapid oxidation of a material (the fuel) in the exothermic chemical process of combustion, releasing heat, light, and various reaction Product (chemistry), products. At a certain point in the combustion reaction, called the ignition point, flames are produced. The ''flame'' is the visible portion of the fire. Flames consist primarily of carbon dioxide, water vapor, oxygen and nitrogen. If hot enough, the gases may become ionized to produce Plasma (physics), plasma. Depending on the substances alight, and any impurities outside, the color of the flame and the fire's Intensity (heat transfer), intensity will be different. Fire in its most common form can result in conflagration, which has the potential to cause physical damage through burning. Fire is an important process that affects ecological systems around the globe. The positive effects of fire include stimulating growth and maintaining various ecological systems. Its negative effects include hazard to life and pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid element. Like all alkali metals, lithium is highly reactive and flammable, and must be stored in vacuum, inert atmosphere, or inert liquid such as purified kerosene or mineral oil. When cut, it exhibits a metallic luster, but moist air corrodes it quickly to a dull silvery gray, then black tarnish. It never occurs freely in nature, but only in (usually ionic) compounds, such as pegmatitic minerals, which were once the main source of lithium. Due to its solubility as an ion, it is present in ocean water and is commonly obtained from brines. Lithium metal is isolated electrolytically from a mixture of lithium chloride and potassium chloride. The nucleus of the lithium atom verges on instability, since the two stable lithium isotopes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
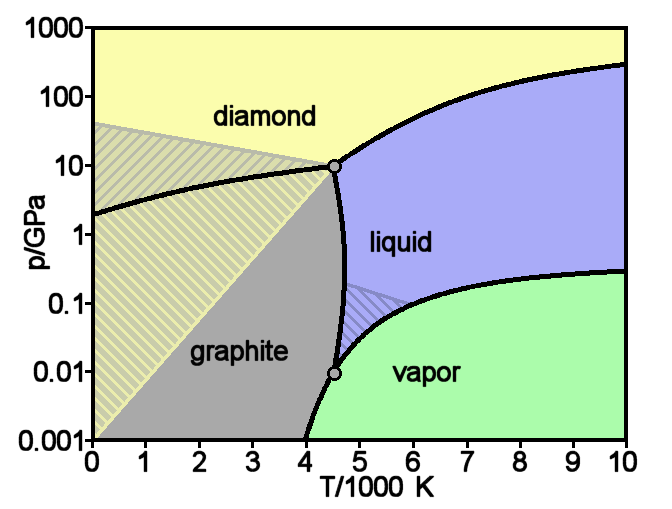
Graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on large scale (300 kton/year, in 1989) for uses in pencils, lubricants, and electrodes. Under high pressures and temperatures it converts to diamond. It is a weak conductor of heat and electricity. Types and varieties Natural graphite The principal types of natural graphite, each occurring in different types of ore deposits, are * Crystalline small flakes of graphite (or flake graphite) occurs as isolated, flat, plate-like particles with hexagonal edges if unbroken. When broken the edges can be irregular or angular; * Amorphous graphite: very fine flake graphite is sometimes called amorphous; * Lump graphite (or vein graphite) occurs in fissure veins or fractures and appears as massive platy intergrowths of fibrous or acicular cry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of NaCl contains 39.34 g Na and 60.66 g Cl. Sodium chloride is the salt most responsible for the salinity of seawater and of the extracellular fluid of many multicellular organisms. In its edible form, salt (also known as '' table salt'') is commonly used as a condiment and food preservative. Large quantities of sodium chloride are used in many industrial processes, and it is a major source of sodium and chlorine compounds used as feedstocks for further chemical syntheses. Another major application of sodium chloride is de-icing of roadways in sub-freezing weather. Uses In addition to the familiar domestic uses of salt, more dominant applications of the approximately 250 million tonnes per year productio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
National Fire Protection Association
The National Fire Protection Association (NFPA) is an international nonprofit organization devoted to eliminating death, injury, property and economic loss due to fire, electrical and related hazards. As of 2018, the NFPA claims to have 50,000 members and 9,000 volunteers working with the organization through its 250 technical committees. History In 1895, a Committee on Automatic Sprinkler Protection was formed in Massachusetts by men affiliated with several fire insurance companies and a pipe manufacturer to develop a uniform standard for the design and installation of fire sprinkler systems. At the time, there were nine such standards in effect within of Boston, Massachusetts, and such diversity was causing great difficulties for plumbers working in the New England New England is a region comprising six states in the Northeastern United States: Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont. It is bordered by the state of New York to th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shavings
Swarf, also known as chips or by other process-specific names (such as turnings, filings, or shavings), are pieces of metal, wood, or plastic that are the debris or waste resulting from machining, woodworking, or similar subtractive (material-removing) manufacturing processes. Swarf or chips can be small particles (such as the gritty swarf from grinding metal or the sawdust from sawing or sanding wood); long, stringy tendrils (such as the springy chips from turning tough metals, or long shavings from whittling); slag-like waste (such as is produced within pipe during pipefitting work); or stone fragments and dust (as in masonry)."The universe is finished; the copestone is on, and the chips were carted off a million years ago." —Ishmael, in ''Moby-Dick'', by Herman Melville. Some of these terms are mass nouns (such as ''swarf'' and ''sawdust'') and some of them are count nouns (such as ''chips'', ''filings'', or ''shavings''). Wood swarf is discussed at ''sawdust''. Meta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sawdust
Sawdust (or wood dust) is a by-product or waste product of woodworking operations such as sawing, sanding, milling, planing, and routing. It is composed of small chippings of wood. These operations can be performed by woodworking machinery, portable power tools or by use of hand tools. Wood dust is also the byproduct of certain animals, birds and insects which live in wood, such as the woodpecker and carpenter ant. In some manufacturing industries it can be a significant fire hazard and source of occupational dust exposure. Sawdust, as particulates, is the main component of particleboard. Research on health hazards comes from the field of occupational safety and health, and study of ventilation happens in indoor air quality engineering. Formation Two waste products, dust and chips, form at the working surface during woodworking operations such as sawing, milling and sanding. These operations both shatter lignified wood cells and break out whole cells and groups of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature, and must be prepared from compounds. Sodium is the sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been leached by the action of water from the Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans. Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide ( lye) is used in soap manufacture, and sodium chloride ( edible salt) is a de-icing agent and a nutrient for animals i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrophoricity
A substance is pyrophoric (from grc-gre, πυροφόρος, , 'fire-bearing') if it ignites spontaneously in air at or below (for gases) or within 5 minutes after coming into contact with air (for liquids and solids). Examples are organolithium compounds and triethylborane. Pyrophoric materials are often water-reactive as well and will ignite when they contact water or humid air. They can be handled safely in atmospheres of argon or (with a few exceptions) nitrogen. Class D fire extinguishers are designated for use in fires involving pyrophoric materials. A related concept is hypergolicity, in which two compounds spontaneously ignite when mixed. Uses The creation of sparks from metals is based on the pyrophoricity of small metal particles, and pyrophoric alloys are made for this purpose. The sparking mechanisms in lighters and various toys, using ferrocerium; starting fires without matches, using a firesteel; the flintlock mechanism in firearms; and spark testing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name ''zirconium'' is taken from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian '' zargun'' (zircon; ''zar-gun'', "gold-like" or "as gold"). It is a lustrous, grey-white, strong transition metal that closely resembles hafnium and, to a lesser extent, titanium. Zirconium is mainly used as a refractory and opacifier, although small amounts are used as an alloying agent for its strong resistance to corrosion. Zirconium forms a variety of inorganic and organometallic compounds such as zirconium dioxide and zirconocene dichloride, respectively. Five isotopes occur naturally, four of which are stable. Zirconium compounds have no known biological role. Characteristics Zirconium is a lustrous, greyish-white, soft, ductile, malleable metal that is solid at room temperature, though it is hard and brittle at lesser purities. In powder form, zirconi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
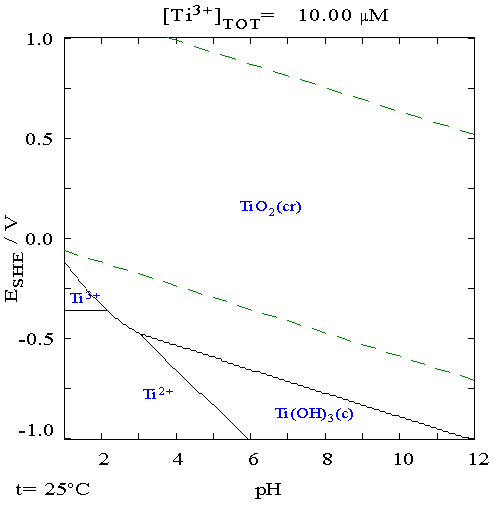
Titanium
Titanium is a chemical element with the Symbol (chemistry), symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in sea water, aqua regia, and chlorine. Titanium was discovered in Cornwall, Kingdom of Great Britain, Great Britain, by William Gregor in 1791 and was named by Martin Heinrich Klaproth after the Titan (mythology), Titans of Greek mythology. The element occurs within a number of minerals, principally rutile and ilmenite, which are widely distributed in the Earth's crust and lithosphere; it is found in almost all living things, as well as bodies of water, rocks, and soils. The metal is extracted from its principal mineral ores by the Kroll process, Kroll and Hunter process, Hunter processes. The most common compound, titanium dioxide, is a popular photocatalysis, photocatalyst and is used in the manufacture of white pigments ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Group 4 Element
Group 4 is the second group of transition metals in the periodic table. It contains the four elements titanium (Ti), zirconium (Zr), hafnium (Hf), and rutherfordium (Rf). The group is also called the titanium group or titanium family after its lightest member. As is typical for early transition metals, zirconium and hafnium have only the group oxidation state of +4 as a major one, and are quite electropositive and have a less rich coordination chemistry. Due to the effects of the lanthanide contraction, they are very similar in properties. Titanium is somewhat distinct due to its smaller size: it has a well-defined +3 state as well (although +4 is more stable). All the group 4 elements are hard, refractory metals. Their inherent reactivity is completely masked due to the formation of a dense oxide layer that protects them from corrosion, as well as attack by many acids and alkalis. The first three of them occur naturally. Rutherfordium is strongly radioactive: it does not occur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




.jpg)