|
FAS-associating Death Domain-containing Protein
FAS-associated death domain protein, also called MORT1, is encoded by the ''FADD'' gene on the 11q13.3 region of chromosome 11 in humans. FADD is an adaptor protein that bridges members of the tumor necrosis factor receptor superfamily, such as the Fas-receptor, to procaspases 8 and 10 to form the death-inducing signaling complex (DISC) during apoptosis. As well as its most well known role in apoptosis, FADD has also been seen to play a role in other processes including proliferation, cell cycle regulation and development. Structure FADD is a 23 kDa protein, made up of 208 amino acids. It contains two main domains: a C terminal death domain (DD) and an N terminal death effector domain (DED). Each domain, although sharing very little sequence similarity, are structurally similar to one another, with each consisting of 6 α helices. The DD of FADD binds to receptors such as the Fas receptor at the plasma membrane via their DD. The interaction between the death domains are e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Signal Transducing Adaptor Protein
Signal transducing adaptor proteins (STAPs) are proteins that are accessory to main proteins in a signal transduction pathway. Adaptor proteins contain a variety of protein-binding modules that link protein-binding partners together and facilitate the creation of larger signaling complexes. These proteins tend to lack any intrinsic enzymatic activity themselves, instead mediating specific protein–protein interactions that drive the formation of protein complexes. Examples of adaptor proteins include MYD88, Grb2 and SHC1. Signaling components Much of the specificity of signal transduction depends on the recruitment of several signalling components such as protein kinases and G-protein GTPases into short-lived active complexes in response to an activating signal such as a growth factor binding to its receptor. Domains Adaptor proteins usually contain several domains within their structure (e.g., Src homology 2 (SH2) and SH3 domains) that allow specific interactions with sev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TNFRSF10A
Death receptor 4 (DR4), also known as TRAIL receptor 1 (TRAILR1) and tumor necrosis factor receptor superfamily member 10A (TNFRSF10A), is a cell surface receptor of the TNF-receptor superfamily that binds TRAIL and mediates apoptosis. Function The protein encoded by this gene is a member of the TNF-receptor superfamily. This receptor is activated by tumor necrosis factor-related apoptosis inducing ligand (TNFSF10/TRAIL), and thus transduces cell death signal and induces cell apoptosis. Studies with FADD-deficient mice suggested that FADD, a death domain containing adaptor protein, is required for the apoptosis mediated by this protein. Interactions TNFRSF10A has been shown to interact with DAP3 28S ribosomal protein S29, mitochondrial, also known as death-associated protein 3 (DAP3), is a protein that in humans is encoded by the ''DAP3'' gene on chromosome 1. This gene encodes a 28S subunit protein of the mitochondrial ribosome (mitori .... References Further reading * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IFN-γ
Interferon gamma (IFN-γ) is a dimerized soluble cytokine that is the only member of the type II class of interferons. The existence of this interferon, which early in its history was known as immune interferon, was described by E. F. Wheelock as a product of human leukocytes stimulated with phytohemagglutinin, and by others as a product of antigen-stimulated lymphocytes. It was also shown to be produced in human lymphocytes. or tuberculin-sensitized mouse peritoneal lymphocytes challenged with Mantoux test (PPD); the resulting supernatants were shown to inhibit growth of vesicular stomatitis virus. Those reports also contained the basic observation underlying the now widely employed IFN-γ release assay used to test for tuberculosis. In humans, the IFN-γ protein is encoded by the ''IFNG'' gene. Through cell signaling, IFN-γ plays a role in regulating the immune response of its target cell. A key signaling pathway that is activated by type II IFN is the JAK-STAT sign ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ATG5
Autophagy related 5 (ATG5) is a protein that, in humans, is encoded by the ''ATG5'' gene located on Chromosome 6. It is an E3 ubi autophagic cell death. ATG5 is a key protein involved in the extension of the phagophoric membrane in autophagic vesicles. It is activated by ATG7 and forms a complex with ATG12 and ATG16L1. This complex is necessary for LC3-I (microtubule-associated proteins 1A/1B light chain 3B) conjugation to PE (phosphatidylethanolamine) to form LC3-II (LC3-phosphatidylethanolamine conjugate). ATG5 can also act as a pro-apoptotic molecule targeted to the mitochondria. Under low levels of DNA damage, ATG5 can translocate to the nucleus and interact with survivin. ATG5 is known to be regulated via various stress induced transcription factors and protein kinases. Structure ATG5 comprises three domains: a ubiquitin-like N-terminal domain (UblA), a helix-rich domain (HR) and a ubiquitin-like C-terminal domain (UblB). The three domains are connected by two linke ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
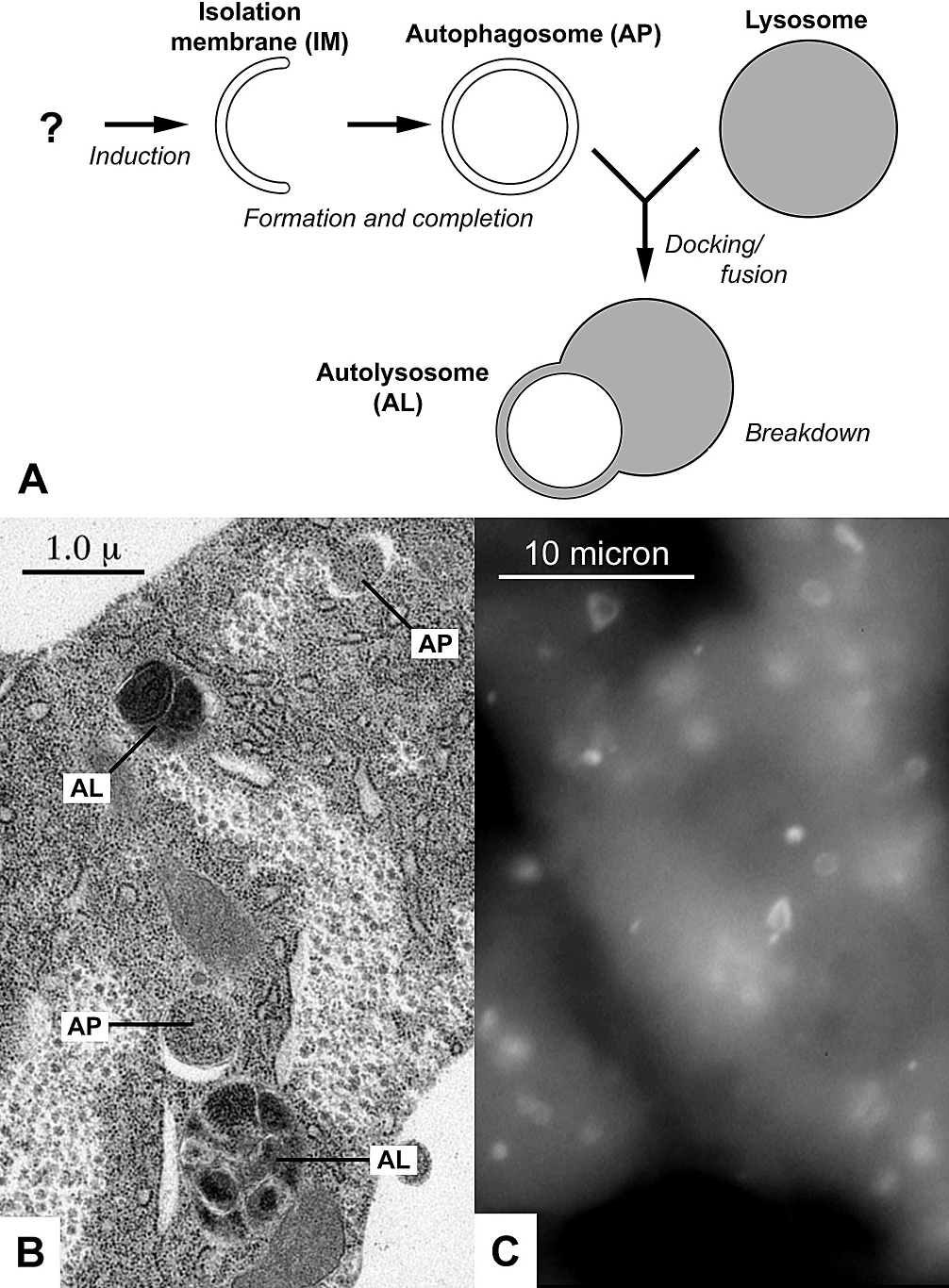
Autophagy
Autophagy (or autophagocytosis; from the Ancient Greek , , meaning "self-devouring" and , , meaning "hollow") is the natural, conserved degradation of the cell that removes unnecessary or dysfunctional components through a lysosome-dependent regulated mechanism. It allows the orderly degradation and recycling of cellular components. Although initially characterized as a primordial degradation pathway induced to protect against starvation, it has become increasingly clear that autophagy also plays a major role in the homeostasis of non-starved cells. Defects in autophagy have been linked to various human diseases, including neurodegeneration and cancer, and interest in modulating autophagy as a potential treatment for these diseases has grown rapidly. Four forms of autophagy have been identified: macroautophagy, microautophagy, chaperone-mediated autophagy (CMA), and crinophagy. In macroautophagy (the most thoroughly researched form of autophagy), cytoplasmic components (like mit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kinases
In biochemistry, a kinase () is an enzyme that catalysis, catalyzes the transfer of phosphate groups from High-energy phosphate, high-energy, phosphate-donating molecules to specific Substrate (biochemistry), substrates. This process is known as phosphorylation, where the high-energy adenosine triphosphate, ATP molecule donates a phosphate group to the substrate (biology), substrate molecule. This transesterification produces a phosphorylated substrate and Adenosine diphosphate, ADP. Conversely, it is referred to as dephosphorylation when the phosphorylated substrate donates a phosphate group and adenosine diphosphate, ADP gains a phosphate group (producing a dephosphorylated substrate and the high energy molecule of ATP). These two processes, phosphorylation and dephosphorylation, occur four times during glycolysis. Kinases are part of the larger family of phosphotransferases. Kinases should not be confused with phosphorylases, which catalyze the addition of inorganic phosphate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RIPK3
Receptor-interacting serine/threonine-protein kinase 3 is an enzyme that is encoded by the ''RIPK3'' gene in humans. The product of this gene is a member of the receptor-interacting protein (RIP) family of serine/threonine protein kinases. It contains a C-terminal domain unique from other RIP family members. The encoded protein is predominantly localized to the cytoplasm, and can undergo nucleocytoplasmic shuttling dependent on novel nuclear localization and export signals. It is a component of the tumor necrosis factor (TNF) receptor-I signaling complex, and can induce necroptosis by interaction with RIPK1 and MLKL in a protein complex termed the necrosome. Interactions between RIPK1 and RIPK3 also form a necrosome, which triggers apoptosis. Interactions RIPK3 has been shown to interact with RIPK1 Receptor-interacting serine/threonine-protein kinase 1 (RIPK1) functions in a variety of cellular pathways related to both cell survival and death. In terms of cell death, RIPK1 pla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RIPK1
Receptor-interacting serine/threonine-protein kinase 1 (RIPK1) functions in a variety of cellular pathways related to both cell survival and death. In terms of cell death, RIPK1 plays a role in apoptosis and necroptosis. Some of the cell survival pathways RIPK1 participates in include NF-κB, Akt, and JNK. RIPK1 is an enzyme that in humans is encoded by the ''RIPK1'' gene, which is located on chromosome 6. This protein belongs to the Receptor Interacting Protein (RIP) kinases family, which consists of 7 members, RIPK1 being the first member of the family. Structure RIPK1 protein is composed of 671 amino acids, and has a molecular weight of about 76 kDa. It contains a serine/threonine kinase domain (KD) in the 300 aa N-Terminus, a death domain (DD) in the 112 aa C-Terminus, and a central region between the KD and DD called intermediate domain (ID). *The kinase domain plays different roles in cell survival and is important in necroptosis induction. RIP interacts with TRAF2 via t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine/threonine-specific Protein Kinase
A serine/threonine protein kinase () is a kinase enzyme, in particular a protein kinase, that phosphorylates the OH group of the amino-acid residues serine or threonine, which have similar side chains. At least 350 of the 500+ human protein kinases are serine/threonine kinases (STK). In enzymology, the term ''serine/threonine protein kinase'' describes a class of enzymes in the family of transferases, that transfer phosphates to the oxygen atom of a serine or threonine side chain in proteins. This process is called phosphorylation. Protein phosphorylation in particular plays a significant role in a wide range of cellular processes and is a very important posttranslational modification. The chemical reaction performed by these enzymes can be written as :ATP + a protein \rightleftharpoons ADP + a phosphoprotein Thus, the two substrates of this enzyme are ATP and a protein, whereas its two products are ADP and phosphoprotein. The systematic name of this enzyme class is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Necroptosis
Necroptosis is a programmed form of necrosis, or inflammatory cell death. Conventionally, necrosis is associated with unprogrammed cell death resulting from cellular damage or infiltration by pathogens, in contrast to orderly, programmed cell death via apoptosis. The discovery of necroptosis showed that cells can execute necrosis in a programmed fashion and that apoptosis is not always the preferred form of cell death. Furthermore, the immunogenic nature of necroptosis favors its participation in certain circumstances, such as aiding in defence against pathogens by the immune system. Necroptosis is well defined as a viral defense mechanism, allowing the cell to undergo "cellular suicide" in a caspase-independent fashion in the presence of viral caspase inhibitors to restrict virus replication. In addition to being a response to disease, necroptosis has also been characterized as a component of inflammatory diseases such as Crohn's disease, pancreatitis, and myocardial infarction. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Extrinsic Apoptosis
In science and engineering, an intrinsic property is a property of a specified subject that exists itself or within the subject. An extrinsic property is not essential or inherent to the subject that is being characterized. For example, mass is an intrinsic property of any physical object, whereas weight is an extrinsic property that depends on the strength of the gravitational field in which the object is placed. Applications in science and engineering In materials science, an intrinsic property is independent of how much of a material is present and is independent of the form of the material, e.g., one large piece or a collection of small particles. Intrinsic properties are dependent mainly on the fundamental chemical composition and structure of the material. Extrinsic properties are differentiated as being dependent on the presence of avoidable chemical contaminants or structural defects. In biology, intrinsic effects originate from inside an organism or cell, such a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |