|
F-Met
''N''-Formylmethionine (fMet, HCO-Met, For-Met) is a derivative of the amino acid methionine in which a formyl group has been added to the amino group. It is specifically used for initiation of protein synthesis from bacterial and organellar genes, and may be removed post-translationally. fMet plays a crucial part in the protein synthesis of bacteria, mitochondria and chloroplasts. It is not used in cytosolic protein synthesis of eukaryotes, where eukaryotic nuclear genes are translated. It is also not used by Archaea. In the human body, fMet is recognized by the immune system as foreign material, or as an alarm signal released by damaged cells, and stimulates the body to fight against potential infection. Function in protein synthesis fMet is a starting residue in the synthesis of proteins in bacteria, and, consequently, is located at the ''N''-terminus of the growing polypeptide. fMet is delivered to the ribosome (30S) - mRNA complex by a specialized tRNA (tRNAfMet) which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Translation (biology)
In molecular biology and genetics, translation is the process in which ribosomes in the cytoplasm or endoplasmic reticulum synthesize proteins after the process of transcription (biology), transcription of DNA to RNA in the cell's nucleus (cell), nucleus. The entire process is called gene expression. In translation, mRNA, messenger RNA (mRNA) is decoded in a ribosome, outside the nucleus, to produce a specific amino acid chain, or polypeptide. The polypeptide later protein folding, folds into an Activation energy, active protein and performs its functions in the Cell (biology), cell. The ribosome facilitates decoding by inducing the binding of Base pair, complementary tRNA anticodon sequences to mRNA codons. The tRNAs carry specific amino acids that are chained together into a polypeptide as the mRNA passes through and is "read" by the ribosome. Translation proceeds in three phases: # Initiation: The ribosome assembles around the target mRNA. The first tRNA is attached a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polypeptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. A polypeptide is a longer, continuous, unbranched peptide chain. Hence, peptides fall under the broad chemical classes of biological polymers and oligomers, alongside nucleic acids, oligosaccharides, polysaccharides, and others. A polypeptide that contains more than approximately 50 amino acids is known as a protein. Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, or to another protein or other macromolecule such as DNA or RNA, or to complex macromolecular assemblies. Amino acids that have been incorporated into peptides are termed residues. A water molecule is released during formation of each amide bond.. All peptides except cyclic peptides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mitochondria
A mitochondrion (; ) is an organelle found in the Cell (biology), cells of most Eukaryotes, such as animals, plants and Fungus, fungi. Mitochondria have a double lipid bilayer, membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is used throughout the cell as a source of chemical energy. They were discovered by Albert von Kölliker in 1857 in the voluntary muscles of insects. The term ''mitochondrion'' was coined by Carl Benda in 1898. The mitochondrion is popularly nicknamed the "powerhouse of the cell", a phrase coined by Philip Siekevitz in a 1957 article of the same name. Some cells in some multicellular organisms lack mitochondria (for example, mature mammalian red blood cells). A large number of unicellular organisms, such as microsporidia, parabasalids and diplomonads, have reduced or transformed their mitochondria into mitosome, other structures. One eukaryote, ''Monocercomonoides'', is known to have completely lost its mitocho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BioEssays
''BioEssays'' is a monthly peer-reviewed review journal covering molecular and cellular biology. Areas covered include genetics, genomics, epigenetics, evolution, developmental biology, neuroscience, human biology, physiology, systems biology, and plant biology. The journal also publishes commentaries on aspects of science communication, education, policy, and current affairs. History The journal was established in December 1984 by founding editor-in-chief William J. Whelan under the auspices of the International Union of Biochemistry and Molecular Biology. Adam S. Wilkins became editor in January 1990. Originally published by ICSU Press and The Company of Biologists, ''BioEssays'' has been published by John Wiley & Sons since January 1998. Andrew Moore became editor-in-chief in August 2008. Kerstin Brachhold is current editor-in-chief. Post-publication commenting ''BioEssays'' offers an article-commenting facility via its website. Topics of particular current attention are of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methionine Aminopeptidase
Methionyl aminopeptidase (, ''methionine aminopeptidase'', ''peptidase M'', ''L-methionine aminopeptidase'', ''MAP'') is an enzyme. This enzyme catalyses the following chemical reaction : Release of N-terminal amino acids, preferentially methionine, from peptides and arylamides This membrane-bound enzymatic activity is present in both prokaryotes and eukaryotes. Proteins possessing this activity include METAP1 and METAP2 Methionine aminopeptidase 2 is an enzyme that in humans is encoded by the ''METAP2'' gene. Methionine aminopeptidase 2, a member of the dimetallohydrolase family, is a cytosolic metalloenzyme that catalyzes the hydrolytic removal of N-terminal m .... References External links * {{Portal bar, Biology, border=no EC 3.4.11 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptide Deformylase
In enzymology, a peptide deformylase () is an enzyme that catalyzes the chemical reaction :H2O + formyl-L-methionyl peptide \rightleftharpoons methionyl peptide + formate Thus, the two substrates of this enzyme are formyl-L-methionyl peptide and H2O, whereas its two products are formate and methionyl peptide. This enzyme belongs to the family of hydrolases, those acting on carbon-nitrogen bonds other than peptide bonds, specifically in linear amides. The systematic name of this enzyme class is formyl-L-methionyl peptide amidohydrolase. Structural studies As of late 2007, 34 structures have been solved for this class of enzymes, with PDB accession codes , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , and . See also *Actinonin Actinonin is a naturally occurring antibacterial agent that has demonstrated anti-tumor activity. Actiononin has been shown to inhibit the enzyme peptide deformylase, which is essential in prokaryote A prokaryote () is a sing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
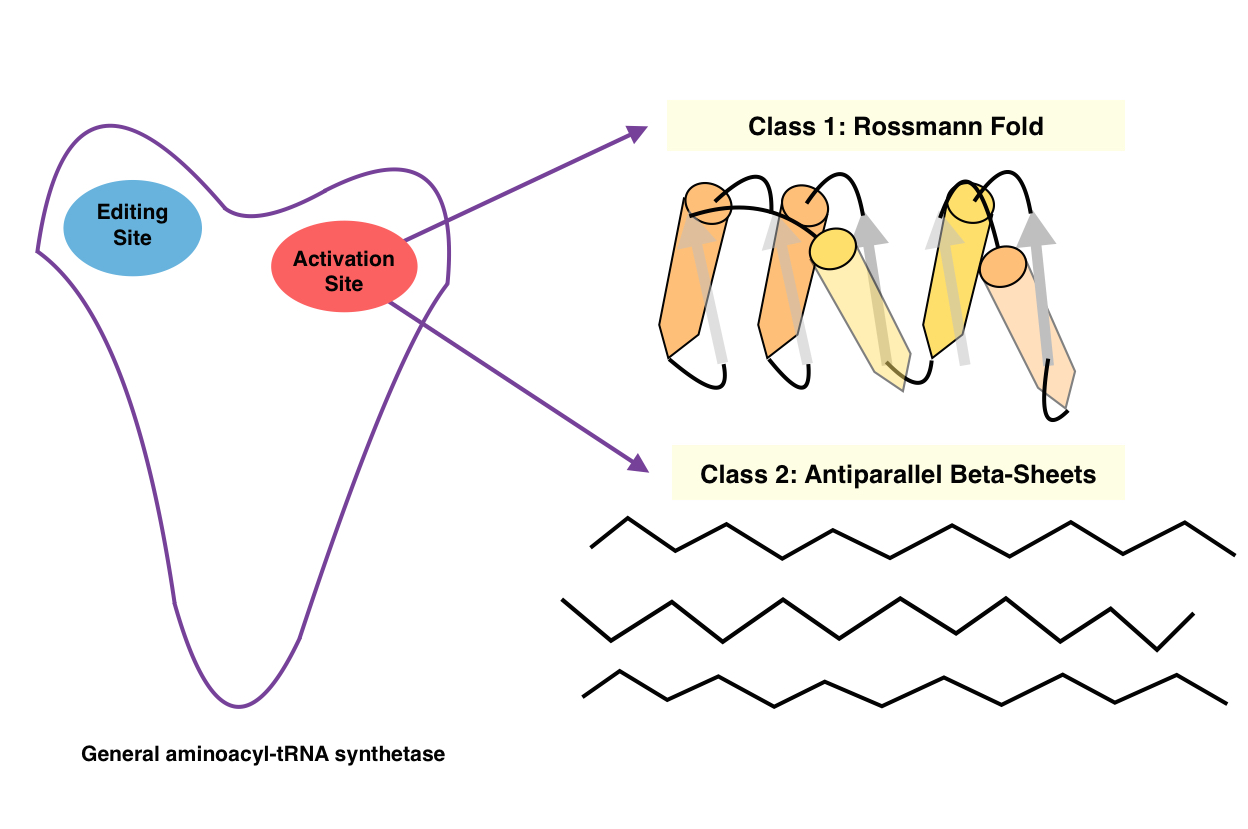
Aminoacyl-tRNA Synthetase
An aminoacyl-tRNA synthetase (aaRS or ARS), also called tRNA-ligase, is an enzyme that attaches the appropriate amino acid onto its corresponding tRNA. It does so by catalyzing the transesterification of a specific cognate amino acid or its precursor to one of all its compatible cognate tRNAs to form an aminoacyl-tRNA. In humans, the 20 different types of aa-tRNA are made by the 20 different aminoacyl-tRNA synthetases, one for each amino acid of the genetic code. This is sometimes called "charging" or "loading" the tRNA with an amino acid. Once the tRNA is charged, a ribosome can transfer the amino acid from the tRNA onto a growing peptide, according to the genetic code. Aminoacyl tRNA therefore plays an important role in RNA translation, the expression of genes to create proteins. Mechanism The synthetase first binds ATP and the corresponding amino acid (or its precursor) to form an aminoacyl-adenylate, releasing inorganic pyrophosphate (PPi). The adenylate-aaRS complex th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methionyl-tRNA Formyltransferase
In enzymology, a methionyl-tRNA formyltransferase () is an enzyme that catalyzes the chemical reaction : 10-formyltetrahydrofolate + L-methionyl-tRNAfMet + H2O \rightleftharpoons tetrahydrofolate + ''N''-formylmethionyl-tRNAfMet This enzyme belongs to the family of transferases that transfer one-carbon groups, specifically the hydroxymethyl-, formyl- and related transferases. The systematic name of this enzyme class is 10-formyltetrahydrofolate:L-methionyl-tRNA N-formyltransferase. Other names in common use include N10-formyltetrahydrofolic-methionyl-transfer ribonucleic, transformylase, formylmethionyl-transfer ribonucleic synthetase, methionyl ribonucleic formyltransferase, methionyl-tRNA Met formyltransferase, methionyl-tRNA transformylase, methionyl-transfer RNA transformylase, methionyl-transfer ribonucleate methyltransferase, and methionyl-transfer ribonucleic transformylase. This enzyme participates in 3 metabolic pathways: methionine metabolism, one carbon pool by fola ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. A polypeptide is a longer, continuous, unbranched peptide chain. Hence, peptides fall under the broad chemical classes of biological polymers and oligomers, alongside nucleic acids, oligosaccharides, polysaccharides, and others. A polypeptide that contains more than approximately 50 amino acids is known as a protein. Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, or to another protein or other macromolecule such as DNA or RNA, or to complex macromolecular assemblies. Amino acids that have been incorporated into peptides are termed residues. A water molecule is released during formation of each amide bond.. All peptides except cyclic pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MRNA
In molecular biology, messenger ribonucleic acid (mRNA) is a single-stranded molecule of RNA that corresponds to the genetic sequence of a gene, and is read by a ribosome in the process of Protein biosynthesis, synthesizing a protein. mRNA is created during the process of Transcription (biology), transcription, where an enzyme (RNA polymerase) converts the gene into primary transcript mRNA (also known as pre-mRNA). This pre-mRNA usually still contains introns, regions that will not go on to code for the final amino acid sequence. These are removed in the process of RNA splicing, leaving only exons, regions that will encode the protein. This exon sequence constitutes mature mRNA. Mature mRNA is then read by the ribosome, and, utilising amino acids carried by transfer RNA (tRNA), the ribosome creates the protein. This process is known as Translation (biology), translation. All of these processes form part of the central dogma of molecular biology, which describes the flow of genet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |