|
Exomer
Exomer is a heterotetrameric protein complex similar to COPI and other adaptins. It was first described in the yeast ''Saccharomyces cerevisiae''. Exomer is a cargo adaptor important in transporting molecules from the Golgi apparatus toward the cell membrane. The vesicles it is found on are different from COPI vesicles in that they do not appear to have a "coat" or "scaffold" around them. An overview of the cellular localization of exomer and other cargo adaptoris shown here Exomer binds to 2 molecules of ADP-ribosylation factor 1 (Arf1) as showin this figure A hinge region of exomer is thought to be important for forming to a highly curved membrane vesicle as showin this figure The steps of assembly of exomer on a Golgi membrane are showin this figure See also * AP2 adaptor complex *Clathrin vesicles *COPII The Coat Protein Complex II, or COPII, is a group of proteins that facilitate the formation of vesicles to transport proteins from the endoplasmic reticulum to the Golg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AP2 Adaptor Complex
The AP2 adaptor complex is a multimeric protein that works on the cell membrane to internalize cargo in clathrin-mediated endocytosis. It is a stable complex of four adaptins which give rise to a structure that has a core domain and two appendage domains attached to the core domain by polypeptide linkers. These appendage domains are sometimes called 'ears'. The core domain binds to the membrane and to cargo destined for internalisation. The alpha and beta appendage domains bind to accessory proteins and to clathrin. Their interactions allow the temporal and spatial regulation of the assembly of clathrin-coated vesicles and their endocytosis. The AP-2 complex is a heterotetramer consisting of two large adaptins (α and β), a medium adaptin (μ), and a small adaptin (σ): * complex 2 ** AP2A1 (α unit 1) ** AP2A2 (α unit 2) ** AP2B1 (β unit) ** AP2M1 (μ unit) ** AP2S1 (σ unit) Structure The AP2 adaptor complex exists in two primary conformations: the open conformation (active ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
COPI
COPI is a coatomer, a protein complex that coats vesicles transporting proteins from the ''cis'' end of the Golgi complex The Golgi apparatus (), also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic cells. Part of the endomembrane system in the cytoplasm, it packages proteins into membrane-bound vesicles insi ... back to the rough endoplasmic reticulum (ER), where they were originally Translation (genetics), synthesized, and between Golgi compartments. This type of transport is ''retrograde transport'', in contrast to the ''anterograde transport'' associated with the COPII protein. The name "COPI" refers to the specific coat protein complex that initiates the budding process on the ''cis''-Golgi membrane. The coat consists of large protein subcomplexes that are made of seven different protein subunits, namely α, β, β', γ, Archain, δ, ε and COPZ1, ζ. Coat proteins Coat protein, or COPI, is an ADP ribosylation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Adaptins
Clathrin adaptor proteins, also known as adaptins, are proteins that mediate the formation of vesicles for intracellular trafficking and secretion. Adaptins are clustered subunits of adaptor protein (AP) complexes. There are several types of adaptin, each related to a different AP complex. Adaptins show sequence similarity to some COPI subunits, thus they are thought to have a common evolutionary origin. The adaptin is a heterotetramer consisting of two large adaptins (beta and one other depending on the complex), a medium adaptin (mu), and a small adaptin (sigma): * complex 1 ** AP1B1 ** AP1G1 ** AP1G2 ** AP1M1 ** AP1M2 ** AP1S1 ** AP1S2 *AP1S3* complex 2 ** AP2A1 ** AP2A2 ** AP2B1 ** AP2M1 ** AP2S1 * complex 3 ** AP3B1 ** AP3B2 ** AP3D1 ** AP3M1 *AP3M2** AP3S1 ** AP3S2 AP-3 complex subunit sigma-2 is a protein that in humans is encoded by the ''AP3S2'' gene. Interactions AP3S2 has been shown to interact Advocates for Informed Choice, doing business as, dba interACT o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yeast
Yeasts are eukaryotic, single-celled microorganisms classified as members of the fungus kingdom. The first yeast originated hundreds of millions of years ago, and at least 1,500 species are currently recognized. They are estimated to constitute 1% of all described fungal species. Yeasts are unicellular organisms that evolved from multicellular ancestors, with some species having the ability to develop multicellular characteristics by forming strings of connected budding cells known as pseudohyphae or false hyphae. Yeast sizes vary greatly, depending on species and environment, typically measuring 3–4 µm in diameter, although some yeasts can grow to 40 µm in size. Most yeasts reproduce asexually by mitosis, and many do so by the asymmetric division process known as budding. With their single-celled growth habit, yeasts can be contrasted with molds, which grow hyphae. Fungal species that can take both forms (depending on temperature or other conditions) are ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saccharomyces Cerevisiae
''Saccharomyces cerevisiae'' () (brewer's yeast or baker's yeast) is a species of yeast (single-celled fungus microorganisms). The species has been instrumental in winemaking, baking, and brewing since ancient times. It is believed to have been originally isolated from the skin of grapes. It is one of the most intensively studied eukaryotic model organisms in molecular biology, molecular and cell biology, much like ''Escherichia coli'' as the model bacteria, bacterium. It is the microorganism behind the most common type of fermentation (biochemistry), fermentation. ''S. cerevisiae'' cells are round to ovoid, 5–10 micrometre, μm in diameter. It reproduces by budding. Many proteins important in human biology were first discovered by studying their Homology (biology), homologs in yeast; these proteins include cell cycle proteins, signaling proteins, and protein-processing enzymes. ''S. cerevisiae'' is currently the only yeast cell known to have Berkeley body, Berkeley bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
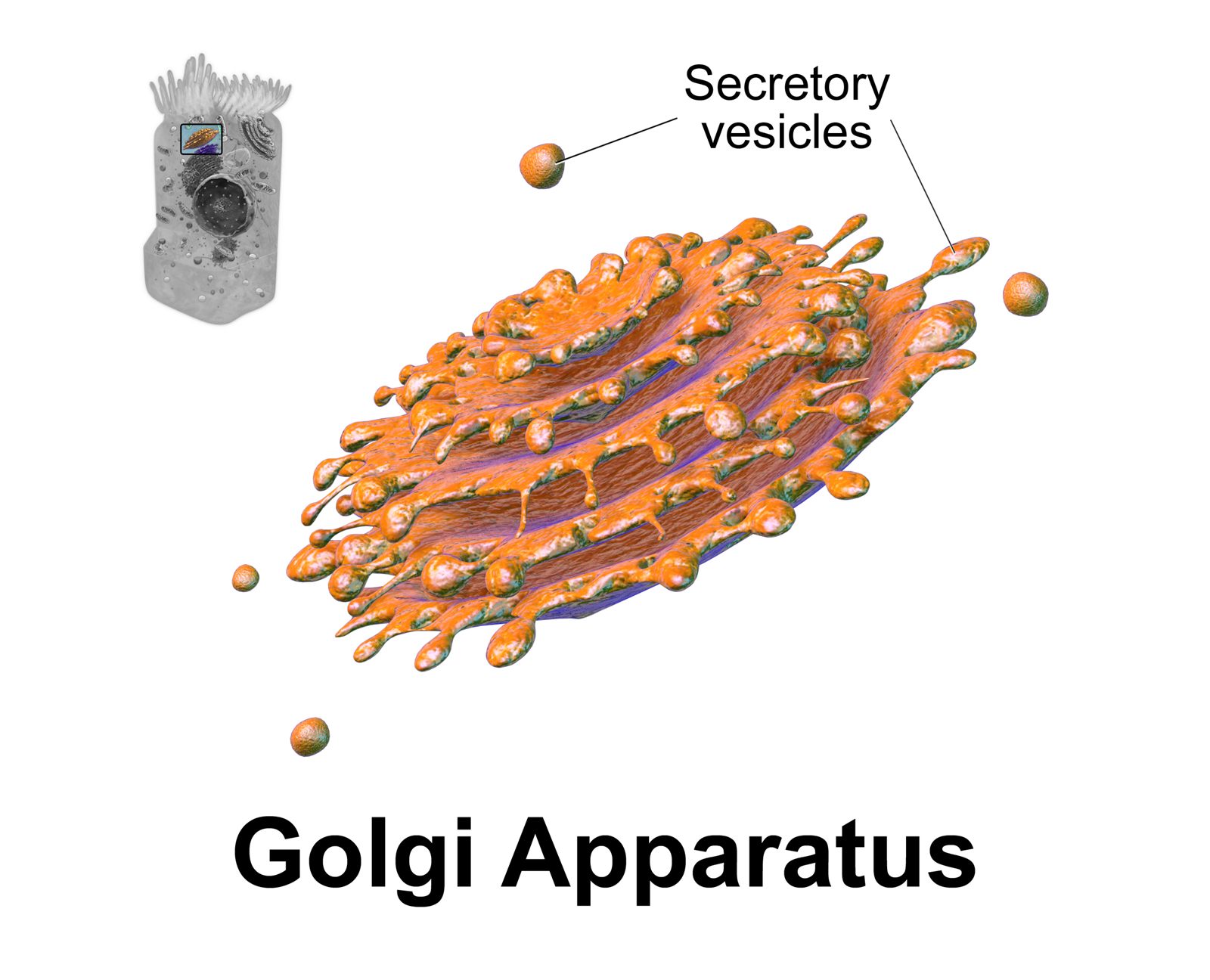
Golgi Apparatus
The Golgi apparatus (), also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic cells. Part of the endomembrane system in the cytoplasm, it packages proteins into membrane-bound vesicles inside the cell before the vesicles are sent to their destination. It resides at the intersection of the secretory, lysosomal, and endocytic pathways. It is of particular importance in processing proteins for secretion, containing a set of glycosylation enzymes that attach various sugar monomers to proteins as the proteins move through the apparatus. It was identified in 1897 by the Italian scientist Camillo Golgi and was named after him in 1898. Discovery Owing to its large size and distinctive structure, the Golgi apparatus was one of the first organelles to be discovered and observed in detail. It was discovered in 1898 by Italian physician Camillo Golgi during an investigation of the nervous system. After first observing it under his ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell Membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment (the extracellular space). The cell membrane consists of a lipid bilayer, made up of two layers of phospholipids with cholesterols (a lipid component) interspersed between them, maintaining appropriate membrane fluidity at various temperatures. The membrane also contains membrane proteins, including integral proteins that span the membrane and serve as membrane transporters, and peripheral proteins that loosely attach to the outer (peripheral) side of the cell membrane, acting as enzymes to facilitate interaction with the cell's environment. Glycolipids embedded in the outer lipid layer serve a similar purpose. The cell membrane controls the movement of substances in and out of cells and organelles, being selectively permeable to ions a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ADP-ribosylation Factor 1
ADP-ribosylation factor 1 is a protein that in humans is encoded by the ''ARF1'' gene. Function ADP-ribosylation factor 1 (ARF1) is a member of the human ARF gene family. The family members encode small guanine nucleotide-binding proteins that stimulate the ADP-ribosyltransferase activity of cholera toxin and play a role in vesicular trafficking as activators of phospholipase D. The gene products, including 6 ARF proteins and 11 ARF-like proteins, constitute a family of the RAS superfamily. The ARF proteins are categorized as class I (ARF1, ARF2 and ARF3), class II (ARF4 and ARF5) and class III (ARF6), and members of each class share a common gene organization. The ARF1 protein is localized to the Golgi apparatus and has a central role in intra-Golgi transport. Multiple alternatively spliced transcript variants encoding the same protein have been found for this gene. The major mechanism of action of Brefeldin A is through inhibition of ARF1. Interactions ARF1 has been shown ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clathrin
Clathrin is a protein that plays a major role in the formation of coated vesicles. Clathrin was first isolated and named by Barbara Pearse in 1976. It forms a triskelion shape composed of three clathrin heavy chains and three light chains. When the triskelia interact they form a polyhedral lattice that surrounds the vesicle, hence the protein's name, which is derived from the Latin ''clathrum'' meaning lattice. Coat-proteins, like clathrin, are used to build small vesicles in order to transport molecules within cells. The endocytosis and exocytosis of vesicles allows cells to communicate, to transfer nutrients, to import signaling receptors, to mediate an immune response after sampling the extracellular world, and to clean up the cell debris left by tissue inflammation. The endocytic pathway can be hijacked by viruses and other pathogens in order to gain entry to the cell during infection. Structure The clathrin triskelion is composed of three clathrin heavy chains inter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
COPII
The Coat Protein Complex II, or COPII, is a group of proteins that facilitate the formation of vesicles to transport proteins from the endoplasmic reticulum to the Golgi apparatus or endoplasmic-reticulum–Golgi intermediate compartment. This process is termed anterograde transport, in contrast to the retrograde transport associated with the COPI complex. COPII is assembled in two parts: first an inner layer of Sar1, Sec23, and Sec24 forms; then the inner coat is surrounded by an outer lattice of Sec13 and Sec31. Function The COPII coat is responsible for the formation of vesicles from the endoplasmic reticulum (ER). These vesicles transport cargo proteins to the Golgi apparatus (in yeast) or the endoplasmic-reticulum-Golgi intermediate compartment (ERGIC, in mammals). Coat assembly is initiated when the cytosolic Ras GTPase Sar1 is activated by its guanine nucleotide exchange factor Sec12. Activated Sar1-GTP inserts itself into the ER membrane, binding preferentially to are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |