|
Ethyl Acetoacetate
The organic compound ethyl acetoacetate (EAA) is the ethyl ester of acetoacetic acid. It is a colorless liquid. It is widely used as a chemical intermediate in the production of a wide variety of compounds. It is used as a flavoring for food. Preparation Ethyl acetoacetate is produced industrially by treatment of diketene with ethanol. The preparation of ethyl acetoacetate is a classic laboratory procedure. It is prepared via the Claisen condensation of ethyl acetate. Two moles of ethyl acetate condense to form one mole each of ethyl acetoacetate and ethanol. : Reactivity Acidity Ethyl acetoacetate is diprotic: :CH3C(O)CH2CO2Et + NaH → CH3C(O)CH(Na)CO2Et + H2 :CH3C(O)CH(Na)CO2Et + BuLi → LiCH2C(O)CH(Na)CO2Et + BuH Keto-enol tautomerism Ethyl acetoacetate is subject to keto-enol tautomerism. In the neat liquid at 33 °C, the enol consists of 15% of the total. Multicarbon building block Ethyl acetoacetic acid is a building block in organic synthesis since the protons alp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fruit
In botany, a fruit is the seed-bearing structure in flowering plants that is formed from the ovary after flowering. Fruits are the means by which flowering plants (also known as angiosperms) disseminate their seeds. Edible fruits in particular have long propagated using the movements of humans and animals in a symbiotic relationship that is the means for seed dispersal for the one group and nutrition for the other; in fact, humans and many animals have become dependent on fruits as a source of food. Consequently, fruits account for a substantial fraction of the world's agricultural output, and some (such as the apple and the pomegranate) have acquired extensive cultural and symbolic meanings. In common language usage, "fruit" normally means the seed-associated fleshy structures (or produce) of plants that typically are sweet or sour and edible in the raw state, such as apples, bananas, grapes, lemons, oranges, and strawberries. In botanical usage, the term "fruit" also i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Acetate
Ethyl acetate ( systematically ethyl ethanoate, commonly abbreviated EtOAc, ETAC or EA) is the organic compound with the formula , simplified to . This colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues, nail polish removers, and in the decaffeination process of tea and coffee. Ethyl acetate is the ester of ethanol and acetic acid; it is manufactured on a large scale for use as a solvent. Production and synthesis Ethyl acetate was first synthesized by the Count de Lauraguais in 1759 by distilling a mixture of ethanol and acetic acid. In 2004, an estimated 1.3 million tonnes were produced worldwide. The combined annual production in 1985 of Japan, North America, and Europe was about 400,000 tonnes. The global ethyl acetate market was valued at $3.3 billion in 2018. Ethyl acetate is synthesized in industry mainly via the classic Fischer esterification reaction of ethanol and acetic acid. This mixture converts to the ester in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Knorr Pyrrole Synthesis
The Knorr pyrrole synthesis is a widely used chemical reaction that synthesizes substituted pyrroles (3). The method involves the reaction of an α-amino-ketone (1) and a compound containing an electron-withdrawing group (e.g. an ester as shown) α to a carbonyl group (2). Method The mechanism requires zinc and acetic acid as catalysts. It will proceed at room temperature. Because α-amino-ketones self-condense very easily, they must be prepared ''in situ''. The usual way of doing this is from the relevant oxime, via the Neber rearrangement. The original Knorr synthesis employed two equivalents of ethyl acetoacetate, one of which was converted to ethyl 2-oximinoacetoacetate by dissolving it in glacial acetic acid, and slowly adding one equivalent of saturated aqueous sodium nitrite, under external cooling. Zinc dust was then stirred in, reducing the oxime group to the amine. This reduction consumes two equivalents of zinc and four equivalents of acetic acid. Modern practice i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing compounds, especially those that include transition metals (elements like titanium that belong to the Periodic Table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom through several of the ligand's atoms; ligands with 2, 3, 4 or even 6 bonds to the central atom are common. These compl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylacetone
Acetylacetone is an organic compound with the chemical formula . It is a colorless liquid, classified as a 1,3-diketone. It exists in equilibrium with a tautomer . These tautomers interconvert so rapidly under most conditions that they are treated as a single compound in most applications. It is a colorless liquid that is a precursor to acetylacetonate anion (commonly abbreviated acac−), a bidentate ligand. It is also a building block for the synthesis of heterocyclic compounds. Properties Tautomerism The keto and enol tautomers of acetylacetone coexist in solution. The enol form has C2v symmetry, meaning the hydrogen atom is shared equally between the two oxygen atoms. In the gas phase, the equilibrium constant, ''K''keto→enol, is 11.7, favoring the enol form. The two tautomeric forms can be distinguished by NMR spectroscopy, IR spectroscopy and other methods. The equilibrium constant tends to be high in nonpolar solvents; when k = >1, the enol form is favour ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carboxylation, the addition of CO2 to a compound. Enzymes that catalyze decarboxylations are called decarboxylases or, the more formal term, carboxy-lyases ( EC number 4.1.1). In organic chemistry The term "decarboxylation" usually means replacement of a carboxyl group () with a hydrogen atom: :RCO2H -> RH + CO2 Decarboxylation is one of the oldest known organic reactions. It is one of the processes assumed to accompany pyrolysis and destructive distillation. Metal salts, especially copper compounds, facilitate the reaction via the intermediacy of metal carboxylate complexes. Decarboxylation of aryl carboxylates can generate the equivalent of the corresponding aryl anion, which in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Knoevenagel Condensation
In organic chemistry, the Knoevenagel condensation () reaction is a type of chemical reaction named after German chemist Emil Knoevenagel. It is a modification of the aldol condensation. A Knoevenagel condensation is a nucleophilic addition of an active hydrogen compound to a carbonyl group followed by a dehydration reaction in which a molecule of water is eliminated (hence ''condensation''). The product is often an α,β-unsaturated ketone (a conjugated enone). In this reaction the carbonyl group is an aldehyde or a ketone. The catalyst is usually a weakly basic amine. The active hydrogen component has the form * or for instance diethyl malonate, Meldrum's acid, ethyl acetoacetate or malonic acid, or cyanoacetic acid. * , for instance nitromethane. where Z is an electron withdrawing group. Z must be powerful enough to facilitate deprotonation to the enolate ion even with a mild base. Using a strong base in this reaction would induce self-condensation of the aldehyde o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Malonic Ester Synthesis
The malonic ester synthesis is a chemical reaction where diethyl malonate or another ester of malonic acid is alkylated at the carbon alpha (directly adjacent) to both carbonyl groups, and then converted to a substituted acetic acid. The major drawback of malonic ester synthesis is that the alkylation stage can also produce dialkylated structures. This makes separation of products difficult and yields lower. : Mechanism The carbons alpha to carbonyl groups can be deprotonated by a strong base. The carbanion formed can undergo nucleophilic substitution on the alkyl halide, to give the alkylated compound. On heating, the di-ester undergoes thermal decarboxylation, yielding an acetic acid substituted by the appropriate R group. Thus, the malonic ester can be thought of being equivalent to the −CH2COOH synthon. The esters chosen are usually the same as the base used, i.e. ethyl esters with sodium ethoxide. This is to prevent scrambling by transesterification. Variations Dialky ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethyl Malonate
Diethyl malonate, also known as DEM, is the diethyl ester of malonic acid. It occurs naturally in grapes and strawberries as a colourless liquid with an apple-like odour, and is used in perfumes. It is also used to synthesize other compounds such as barbiturates, artificial flavourings, vitamin B1, and vitamin B6. Structure and properties Malonic acid is a rather simple dicarboxylic acid, with two the carboxyl groups close together. In forming diethyl malonate from malonic acid, the hydroxyl group (−OH) on both of the carboxyl groups is replaced by an ethoxy group (−OEt; −OCH2CH3). The methylene group (−CH2−) in the middle of the malonic part of the diethyl malonate molecule is neighboured by two carbonyl groups (−C(=O)−). The hydrogen atoms on the carbon adjacent to the carbonyl group in a molecule is significantly more acidic than hydrogen atoms on a carbon adjacent to alkyl groups (up to 30 orders of magnitude). (This is known as the α position with re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
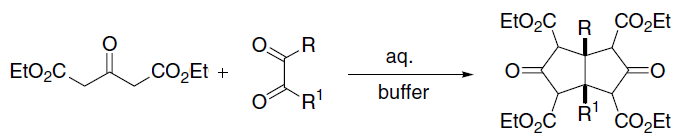
Acetoacetic Ester Synthesis
Acetoacetic ester synthesis is a chemical reaction where ethyl acetoacetate is alkylated at the α-carbon to both carbonyl groups and then converted into a ketone, or more specifically an α-substituted acetone. This is very similar to malonic ester synthesis. : Mechanism A strong base deprotonates the dicarbonyl α-carbon. This carbon is preferred over the methyl carbon because the formed enolate is conjugated and thus resonance stabilized. The carbon then undergoes nucleophilic substitution. When heated with aqueous acid, the newly alkylated ester is hydrolyzed to a β-keto acid, which is decarboxylated to form a methyl ketone. Double deprotonation of ethyl acetoacetate The classical acetoacetatic ester synthesis utilizes the 1:1 conjugate base. Ethyl acetoacetate is however diprotic: :CH3C(O)CH2CO2Et + NaH → CH3C(O)CH(Na)CO2Et + H2 :CH3C(O)CH(Na)CO2Et + BuLi → LiCH2C(O)CH(Na)CO2Et + BuH The dianion (i.e., LiCH2C(O)CH(Na)CO2Et) adds electrophile to the ter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
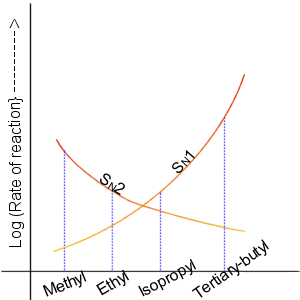
Nucleophilic Substitution
In chemistry, a nucleophilic substitution is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile). The molecule that contains the electrophile and the leaving functional group is called the substrate. The most general form of the reaction may be given as the following: :\text\mathbf + \ce + \text\mathbf The electron pair (:) from the nucleophile (Nuc) attacks the substrate () and bonds with it. Simultaneously, the leaving group (LG) departs with an electron pair. The principal product in this case is . The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged. An example of nucleophilic substitution is the hydrolysis of an alkyl bromide, R-Br under basic conditions, where the attacking nucleophile is hydroxyl () and the leaving group is bromide (). :R-Br + OH- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |