|
Entropy And Life
Research concerning the relationship between the thermodynamic quantity entropy and the evolution of life began around the turn of the 20th century. In 1910, American historian Henry Adams printed and distributed to university libraries and history professors the small volume ''A Letter to American Teachers of History'' proposing a theory of history based on the second law of thermodynamics and on the principle of entropy. The 1944 book ''What is Life?'' by Nobel-laureate physicist Erwin Schrödinger stimulated further research in the field. In his book, Schrödinger originally stated that life feeds on negative entropy, or negentropy as it is sometimes called, but in a later edition corrected himself in response to complaints and stated that the true source is free energy. More recent work has restricted the discussion to Gibbs free energy because biological processes on Earth normally occur at a constant temperature and pressure, such as in the atmosphere or at the bottom of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering and mechanical engineering, but also in other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the efficiency of early steam engines, particularly through the work of French physicist Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win the Napoleonic Wars. Scots-Irish physicist Lord Kelvin was the first to formulate a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rudolf Clausius
Rudolf Julius Emanuel Clausius (; 2 January 1822 – 24 August 1888) was a German physicist and mathematician and is considered one of the central founding fathers of the science of thermodynamics. By his restatement of Sadi Carnot's principle known as the Carnot cycle, he gave the theory of heat a truer and sounder basis. His most important paper, "On the Moving Force of Heat", published in 1850, first stated the basic ideas of the second law of thermodynamics. In 1865 he introduced the concept of entropy. In 1870 he introduced the virial theorem, which applied to heat. Life Clausius was born in Köslin (now Koszalin, Poland) in the Province of Pomerania in Prussia. His father was a Protestant pastor and school inspector, and Rudolf studied in the school of his father. In 1838, he went to the Gymnasium in Stettin. Clausius graduated from the University of Berlin in 1844 where he had studied mathematics and physics since 1840 with, among others, Gustav Magnus, Peter Gustav Le ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
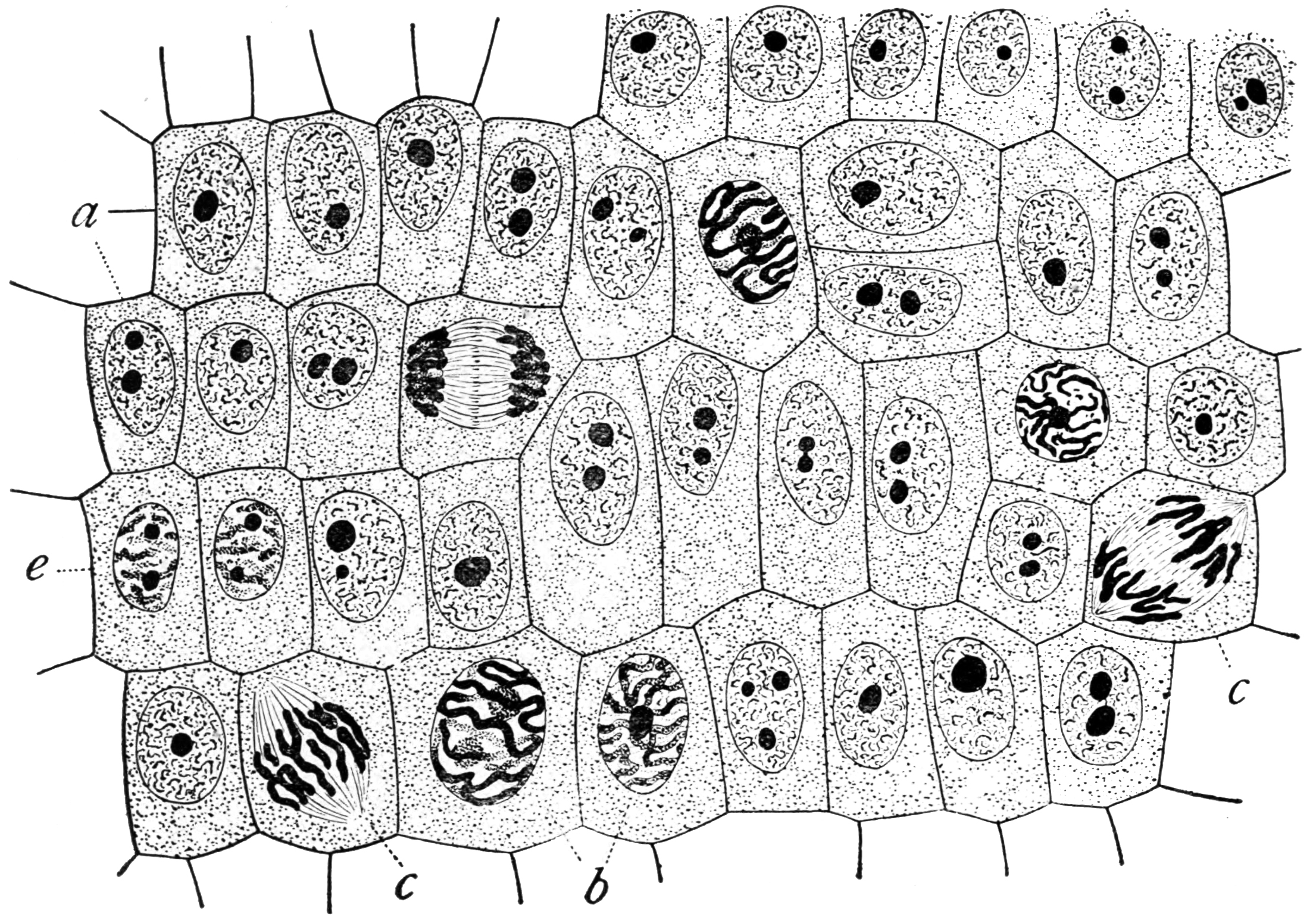
Physiology
Physiology (; ) is the scientific study of functions and mechanisms in a living system. As a sub-discipline of biology, physiology focuses on how organisms, organ systems, individual organs, cells, and biomolecules carry out the chemical and physical functions in a living system. According to the classes of organisms, the field can be divided into medical physiology, animal physiology, plant physiology, cell physiology, and comparative physiology. Central to physiological functioning are biophysical and biochemical processes, homeostatic control mechanisms, and communication between cells. ''Physiological state'' is the condition of normal function. In contrast, ''pathological state'' refers to abnormal conditions, including human diseases. The Nobel Prize in Physiology or Medicine is awarded by the Royal Swedish Academy of Sciences for exceptional scientific achievements in physiology related to the field of medicine. Foundations Cells Although there are differ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Theory Of Heat
The history of thermodynamics is a fundamental strand in the history of physics, the history of chemistry, and the history of science in general. Owing to the relevance of thermodynamics in much of science and technology, its history is finely woven with the developments of classical mechanics, quantum mechanics, magnetism, and chemical kinetics, to more distant applied fields such as meteorology, information theory, and biology (physiology), and to technological developments such as the steam engine, internal combustion engine, cryogenics and electricity generation. The development of thermodynamics both drove and was driven by atomic theory. It also, albeit in a subtle manner, motivated new directions in probability and statistics; see, for example, the timeline of thermodynamics. History Contributions from antiquity The ancients viewed heat as that related to fire. In 3000 BC, the ancient Egyptians viewed heat as related to origin mythologies. The ancient Indian philosophy in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Natural Science
Natural science is one of the branches of science concerned with the description, understanding and prediction of natural phenomena, based on empirical evidence from observation and experimentation. Mechanisms such as peer review and repeatability of findings are used to try to ensure the validity of scientific advances. Natural science can be divided into two main branches: life science and physical science. Life science is alternatively known as biology, and physical science is subdivided into branches: physics, chemistry, earth science, and astronomy. These branches of natural science may be further divided into more specialized branches (also known as fields). As empirical sciences, natural sciences use tools from the formal sciences, such as mathematics and logic, converting information about nature into measurements which can be explained as clear statements of the " laws of nature". Modern natural science succeeded more classical approaches to natural philosophy, usu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
First Law Of Thermodynamics
The first law of thermodynamics is a formulation of the law of conservation of energy, adapted for thermodynamic processes. It distinguishes in principle two forms of energy transfer, heat and thermodynamic work for a system of a constant amount of matter. The law also defines the internal energy of a system, an extensive property for taking account of the balance of energies in the system. The law of conservation of energy states that the total energy of any isolated system, which cannot exchange energy or matter, is constant. Energy can be transformed from one form to another, but can be neither created nor destroyed. The first law for a thermodynamic process is often formulated asThe sign convention (Q is heat supplied ''to'' the system but W is work done ''by'' the system) is that of Rudolf Clausius (Equation IIa on page 384 of Clausius, R. (1850)), and it is followed below. :\Delta U = Q - W, where \Delta U denotes the change in the internal energy of a closed system (f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Temperature
Thermodynamic temperature is a quantity defined in thermodynamics as distinct from kinetic theory or statistical mechanics. Historically, thermodynamic temperature was defined by Kelvin in terms of a macroscopic relation between thermodynamic work and heat transfer as defined in thermodynamics, but the kelvin was redefined by international agreement in 2019 in terms of phenomena that are now understood as manifestations of the kinetic energy of free motion of microscopic particles such as atoms, molecules, and electrons. From the thermodynamic viewpoint, for historical reasons, because of how it is defined and measured, this microscopic kinetic definition is regarded as an "empirical" temperature. It was adopted because in practice it can generally be measured more precisely than can Kelvin's thermodynamic temperature. A thermodynamic temperature reading of zero is of particular importance for the third law of thermodynamics. By convention, it is reported on the ''Kelvin scale'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
System (thermodynamics)
A thermodynamic system is a body of matter and/or radiation, confined in space by walls, with defined permeabilities, which separate it from its surroundings. The surroundings may include other thermodynamic systems, or physical systems that are not thermodynamic systems. A wall of a thermodynamic system may be purely notional, when it is described as being 'permeable' to all matter, all radiation, and all forces. A state of a thermodynamic system can be fully described in several different ways, by several different sets of thermodynamic state variables. A widely used distinction is between ''isolated'', ''closed'', and ''open'' thermodynamic systems. An isolated thermodynamic system has walls that are non-conductive of heat and perfectly reflective of all radiation, that are rigid and immovable, and that are impermeable to all forms of matter and all forces. (Some writers use the word 'closed' when here the word 'isolated' is being used.) A closed thermodynamic system is c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat
In thermodynamics, heat is defined as the form of energy crossing the boundary of a thermodynamic system by virtue of a temperature difference across the boundary. A thermodynamic system does not ''contain'' heat. Nevertheless, the term is also often used to refer to the thermal energy contained in a system as a component of its internal energy and that is reflected in the temperature of the system. For both uses of the term, heat is a form of energy. An example of formal vs. informal usage may be obtained from the right-hand photo, in which the metal bar is "conducting heat" from its hot end to its cold end, but if the metal bar is considered a thermodynamic system, then the energy flowing within the metal bar is called internal energy, not heat. The hot metal bar is also transferring heat to its surroundings, a correct statement for both the strict and loose meanings of ''heat''. Another example of informal usage is the term '' heat content'', used despite the fact that p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nicolas Léonard Sadi Carnot
''Sous-lieutenant'' Nicolas Léonard Sadi Carnot (; 1 June 1796 – 24 August 1832) was a French mechanical engineer in the French Army, military scientist and physicist, and often described as the "father of thermodynamics". He published only one book, the ''Reflections on the Motive Power of Fire'' (Paris, 1824), in which he expressed the first successful theory of the maximum efficiency of heat engines and laid the foundations of the new discipline: thermodynamics. Carnot's work attracted little attention during his lifetime, but it was later used by Rudolf Clausius and Lord Kelvin to formalize the second law of thermodynamics and define the concept of entropy. Based on purely technical concerns, such as improving the performance of the steam engine, Sadi Carnot's intellect laid the groundwork for modern science technological designs, such as the automobile or jet engine. His father Lazare Carnot was an eminent mathematician, military engineer, and leader of the French Revo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
James Prescott Joule
James Prescott Joule (; 24 December 1818 11 October 1889) was an English physicist, mathematician and brewer, born in Salford, Lancashire. Joule studied the nature of heat, and discovered its relationship to mechanical work (see energy). This led to the law of conservation of energy, which in turn led to the development of the first law of thermodynamics. The SI derived unit of energy, the joule, is named after him. He worked with Lord Kelvin to develop an absolute thermodynamic temperature scale, which came to be called the Kelvin scale. Joule also made observations of magnetostriction, and he found the relationship between the current through a resistor and the heat dissipated, which is also called Joule's first law. His experiments about energy transformations were first published in 1843. Early years James Joule was born in 1818, the son of Benjamin Joule (1784–1858), a wealthy brewer, and his wife, Alice Prescott, on New Bailey Street in Salford. Joule was tutored a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Richard Sears McCulloh
Richard Sears McCulloh (18 March 1818 – 1894) was an American civil engineer and professor of mechanics and thermodynamics at the Washington and Lee University, Lexington, Virginia. Career McCulloh was born on 18 March 1818 in Baltimore, Maryland, United States. He graduated from the College of New Jersey in 1836, then studied chemistry in Philadelphia with James Curtis Booth from 1838 to 1839. From 1846 to 1849 he worked for the U.S. Mint in Philadelphia. He was elected to the American Philosophical Society in 1846. McCulloh was appointed professor of natural philosophy at Princeton University on 24 October 1849, and then professor of natural and experimental philosophy at Columbia College on 3 April 1854. During the American Civil War, McCulloh disappeared from New York after the draft riots and in October 1863 McCulloh went to Richmond, Virginia to become the consulting chemist of the Confederate Nitre and Mining Bureau. In response, Columbia College expelled him from his pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |