|
Ene Reaction
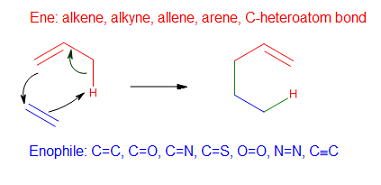
In organic chemistry, the ene reaction (also known as the Alder-ene reaction by its discoverer Kurt Alder in 1943) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile), in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the allylic position. This transformation is a group transfer pericyclic reaction, and therefore, usually requires highly activated substrates and/or high temperatures. Nonetheless, the reaction is compatible with a wide variety of functional groups that can be appended to the ene and enophile moieties. Many useful Lewis acid-catalyzed ene reactions have been also developed, which can afford high yields and selectivities at significantly lower temperatures, making the ene reaction a useful C–C forming tool for the synthesis of complex molecules and natural products. Ene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical ( in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (included in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cumulene
In organic chemistry, a cumulene is a compound having three or more ''cumulative'' (consecutive) double bonds. They are analogous to allenes, only having a more extensive chain. The simplest molecule in this class is butatriene (), which is also called simply ''cumulene''. Unlike most alkanes and alkenes, cumulenes tend to be rigid, comparable to polyynes. Cumulene carbenes for ''n'' from 3 to 6 have been observed in interstellar molecular clouds and in laboratory experiments by using microwave and infrared spectroscopy. (The more stable cumulenes are difficult to detect optically because they lack an electric dipole moment.) Cumulenes containing heteroatoms are called heterocumulenes; an example is carbon suboxide. Synthesis The first reported synthesis of a butatriene is that of tetraphenylbutatriene in 1921. The most common synthetic method for butatriene synthesis is based on reductive coupling of a geminal dihalovinylidene. Tetraphenylbutatriene was reported synthesized ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the Multiplicity (chemistry)#Molecules, bond multiplicity". The resulting reaction is a cyclization reaction. Many but not all cycloadditions are Concerted reaction, concerted and thus pericyclic. Nonconcerted cycloadditions are not pericyclic. As a class of addition reaction, cycloadditions permit carbon–carbon bond formation without the use of a nucleophile or electrophile. Cycloadditions can be described using two systems of notation. An older but still common notation is based on the size of linear arrangements of atoms in the reactants. It uses parentheses: where the variables are the numbers of linear atoms in each reactant. The product is a cycle of size . In this system, the standard Diels-Alder reaction is a (4 + 2)-cyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexene
Cyclohexene is a hydrocarbon with the formula C6H10. This cycloalkene is a colorless liquid with a sharp smell. It is an intermediate in various industrial processes. Cyclohexene is not very stable upon long term storage with exposure to light and air because it forms peroxides. Production and uses Cyclohexene is produced by the partial hydrogenation of benzene, a process developed by the Asahi Chemical company. In the laboratory, it can be prepared by dehydration of cyclohexanol. : : Reactions and uses Benzene is converted to cyclohexylbenzene by acid-catalyzed alkylation with cyclohexene. Cyclohexylbenzene is a precursor to both phenol and cyclohexanone. Hydration of cyclohexene gives cyclohexanol, which can be dehydrogenated to give cyclohexanone, a precursor to caprolactam. The oxidative cleavage of cyclohexene gives adipic acid. Hydrogen peroxide is used as the oxidant in the presence of a tungsten catalyst. Bromination gives 1,2-dibromocyclohexane. Structure Cyclohex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentene
Cyclopentene is a chemical compound with the formula . It is a colorless liquid with a petrol-like odor. It has few applications, and thus is mainly used as a minor component of gasoline, present in concentrations of less than 1%. It is one of the principal cycloalkenes. Production Cyclopentene is produced industrially in large amounts by steam cracking of naphtha. In the laboratory, it is prepared by dehydration of cyclopentanol. It can also produced by the catalytic hydrogenation of cyclopentadiene.D. Hönicke, R. Födisch, P. Claus, M. Olson: ''Cyclopentadiene and Cyclopentene'', in: '' Ullmanns Enzyklopädie der Technischen Chemie'' 2002, Wiley-VCH, Weinheim. Use in mechanistic organic chemistry Cyclopentene is used in analysing the mechanisms of organic reactions. It can be obtained from vinylcyclopropane in the vinylcyclopropane-cyclopentene rearrangement The vinylcyclopropane rearrangement or vinylcyclopropane-cyclopentene rearrangement is a ring expansion reaction, c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethyl Azodicarboxylate
Diethyl azodicarboxylate, conventionally abbreviated as DEAD and sometimes as DEADCAT, is an organic compound with the structural formula CH3CH2O2CN=NCO2CH2CH3. Its molecular structure consists of a central azo functional group, RN=NR, flanked by two ethyl ester groups. This orange-red liquid is a valuable reagent but also quite dangerous and explodes upon heating. Therefore, commercial shipment of pure diethyl azodicarboxylate is prohibited in the United States and is carried out either in solution or on polystyrene particles. DEAD is an aza- dienophile and an efficient dehydrogenating agent, converting alcohols to aldehydes, thiols to di sulfides and hydrazo groups to azo groups; it is also a good electron acceptor. While DEAD is used in numerous chemical reactions it is mostly known as a key component of the Mitsunobu reaction, a common strategy for the preparation of an amine, azide, ether, thioether, or ester from the corresponding alcohol. It is used in the synthesis of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radical (chemistry)
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron. With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes. A notable example of a radical is the hydroxyl radical (HO·), a molecule that has one unpaired electron on the oxygen atom. Two other examples are triplet oxygen and triplet carbene (꞉) which have two unpaired electrons. Radicals may be generated in a number of ways, but typical methods involve redox reactions. Ionizing radiation, heat, electrical discharges, and electrolysis are known to produce radicals. Radicals are intermediates in many chemical reactions, more so than is apparent from the balanced equations. Radicals are important in combustion, atmospheric chemistry, polymerization, plasma chemistry, biochemistry, and many other chemical processes. A majority of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Figure 3new
Figure may refer to: General *A shape, drawing, depiction, or geometric configuration *Figure (wood), wood appearance *Figure (music), distinguished from musical motif *Noise figure, in telecommunication *Dance figure, an elementary dance pattern *A person's figure, human physical appearance Arts *Figurine, a miniature statuette representation of a creature *Action figure, a posable jointed solid plastic character figurine *Figure painting, realistic representation, especially of the human form *Figure drawing *Model figure, a scale model of a creature Writing *figure, in writing, a type of floating block (text, table, or graphic separate from the main text) * Figure of speech, also called a rhetorical figure *Christ figure, a type of character * in typesetting, text figures and lining figures Accounting *Figure, a synonym for number *Significant figures in a decimal number Science * Figure of the Earth, the size and shape of the Earth in geodesy Sports *Figure (horse), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |