|
Extended Hückel Method
The extended Hückel method is a semiempirical quantum chemistry method, developed by Roald Hoffmann since 1963. It is based on the Hückel method but, while the original Hückel method only considers pi orbitals, the extended method also includes the sigma orbitals. The extended Hückel method can be used for determining the molecular orbitals, but it is not very successful in determining the structural geometry of an organic molecule. It can however determine the relative energy of different geometrical configurations. It involves calculations of the electronic interactions in a rather simple way for which the electron-electron repulsions are not explicitly included and the total energy is just a sum of terms for each electron in the molecule. The off-diagonal Hamiltonian matrix elements are given by an approximation due to Wolfsberg and Helmholz that relates them to the diagonal elements and the overlap matrix element. H_ = KS_ \dfrac ''K'' is the Wolfsberg–Helmholz c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Semiempirical Quantum Chemistry Method
Semi-empirical quantum chemistry methods are based on the Hartree–Fock formalism, but make many approximations and obtain some parameters from empirical data. They are very important in computational chemistry for treating large molecules where the full Hartree–Fock method without the approximations is too expensive. The use of empirical parameters appears to allow some inclusion of electron correlation effects into the methods. Within the framework of Hartree–Fock calculations, some pieces of information (such as two-electron integrals) are sometimes approximated or completely omitted. In order to correct for this loss, semi-empirical methods are parametrized, that is their results are fitted by a set of parameters, normally in such a way as to produce results that best agree with experimental data, but sometimes to agree with ''ab initio'' results. Type of simplifications used Semi-empirical methods follow what are often called empirical methods where the two-electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ab Initio Quantum Chemistry Methods
''Ab initio'' quantum chemistry methods are computational chemistry methods based on quantum chemistry. The term was first used in quantum chemistry by Robert Parr and coworkers, including David Craig in a semiempirical study on the excited states of benzene. The background is described by Parr. ''Ab initio'' means "from first principles" or "from the beginning", implying that the only inputs into an ''ab initio'' calculation are physical constants. ''Ab initio'' quantum chemistry methods attempt to solve the electronic Schrödinger equation given the positions of the nuclei and the number of electrons in order to yield useful information such as electron densities, energies and other properties of the system. The ability to run these calculations has enabled theoretical chemists to solve a range of problems and their importance is highlighted by the awarding of the Nobel prize to John Pople and Walter Kohn. Accuracy and scaling ''Ab initio'' electronic structure method ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide (metal carbonyls), cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term " metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep mantle remain active areas of investigation. Some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon ( graphite, diamond, buckminsterfullerene, etc.), carbon monoxide, carbon dioxide, carbides, and the following salts of inorganic anions: carbonates, cyanides, cyanates, and thiocyanates. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it does not occur within living things. History Friedrich Wöhler's conversion of ammonium cyanate into urea in 1828 is often cited as the starting point ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity, the more an atom or a substituent group attracts electrons. Electronegativity serves as a simple way to quantitatively estimate the bond energy, and the sign and magnitude of a bond's chemical polarity, which characterizes a bond along the continuous scale from covalent to ionic bonding. The loosely defined term electropositivity is the opposite of electronegativity: it characterizes an element's tendency to donate valence electrons. On the most basic level, electronegativity is determined by factors like the nuclear charge (the more protons an atom has, the more "pull" it will have on electrons) and the number and lo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
William Lipscomb
William Nunn Lipscomb Jr. (December 9, 1919April 14, 2011) was a Nobel Prize-winning American inorganic and organic chemist working in nuclear magnetic resonance, theoretical chemistry, boron chemistry, and biochemistry. Biography Overview Lipscomb was born in Cleveland, Ohio. His family moved to Lexington, Kentucky in 1920, and he lived there until he received his Bachelor of Science degree in Chemistry at the University of Kentucky in 1941. He went on to earn his Doctor of Philosophy degree in Chemistry from the California Institute of Technology (Caltech) in 1946. From 1946 to 1959 he taught at the University of Minnesota. From 1959 to 1990 he was a professor of chemistry at Harvard University, where he was a professor emeritus since 1990. Lipscomb was married to the former Mary Adele Sargent from 1944 to 1983. They had three children, one of whom lived only a few hours. He married Jean Evans in 1983. They had one adopted daughter. Lipscomb resided in Cambridge, Massa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Woodward–Hoffmann Rules
The Woodward–Hoffmann rules (or the pericyclic selection rules), devised by Robert Burns Woodward and Roald Hoffmann, are a set of rules used to rationalize or predict certain aspects of the stereochemistry and activation energy of pericyclic reactions, an important class of reactions in organic chemistry. The rules are best understood in terms of the concept of ''the conservation of orbital symmetry'' using ''orbital correlation diagrams'' (see Section 3 below). The Woodward–Hoffmann rules are a consequence of the changes in electronic structure that occur during a pericyclic reaction and are predicated on the phasing of the interacting molecular orbitals. They are applicable to all classes of pericyclic reactions (and their microscopic reverse 'retro' processes), including (1) electrocyclizations, (2) cycloadditions, (3) sigmatropic reactions, (4) group transfer reactions, (5) ene reactions, (6) cheletropic reactions, and (7) dyotropic reactions. Due to their elegance, simp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs. A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of an overall chemical reaction. The detailed steps of a reaction are not observable in most cases. The conjectured mechanism is chosen because it is thermodynamically feasible, and has experimental support in isolated intermediates (see next section) or other quantitative and qualitative characteristics of the reaction. It also describes each reactive intermediate, activated complex, and transition state, and which bonds are broken (and in what order), and which bonds are formed (and in what order). A complete mechanism must also explain the reason for the reactants and catalyst used, the stereochemistry observed in reactants and products, all products formed and the amount of each. The electron or arrow pushing method is often used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
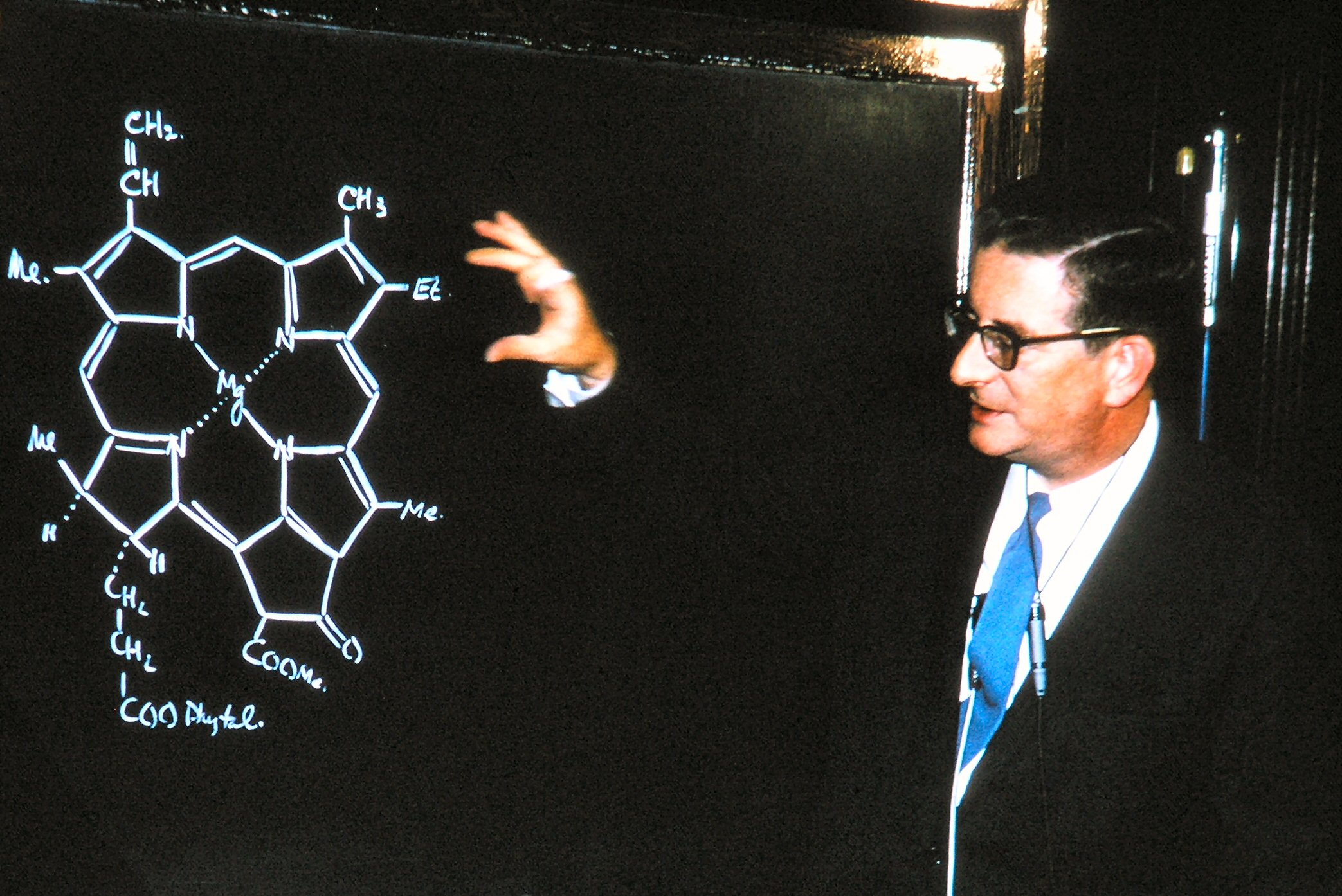
Robert Burns Woodward
Robert Burns Woodward (April 10, 1917 – July 8, 1979) was an American organic chemist. He is considered by many to be the most preeminent synthetic organic chemist of the twentieth century, having made many key contributions to the subject, especially in the synthesis of complex natural products and the determination of their molecular structure. He also worked closely with Roald Hoffmann on theoretical studies of chemical reactions. He was awarded the Nobel Prize in Chemistry in 1965. Early life and education Woodward was born in Boston, Massachusetts, on April 10, 1917. He was the son of Margaret Burns (an immigrant from Scotland who claimed to be a descendant of the poet, Robert Burns) and her husband, Arthur Chester Woodward, himself the son of Roxbury apothecary, Harlow Elliot Woodward. His father was one of the many victims of the 1918 influenza pandemic of 1918. From a very early age, Woodward was attracted to and engaged in private study of chemistry while he at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CNDO/2
CNDO is the abbreviation for Complete Neglect of Differential Overlap, one of the first semi empirical methods in quantum chemistry. It uses two approximations: *core approximation - only the outer valence electrons are explicitly included. * zero-differential overlap CNDO/2 is the main version of CNDO. The method was first introduced by John Pople and coworkers. Background An earlier method was Extended Hückel method, which explicitly ignores electron-electron repulsion terms. It was a method for calculating the electronic energy and the molecular orbitals. CNDO/1 and CNDO/2 were developed from this method by explicitly including the electron-electron repulsion terms, but neglecting many of them, approximating some of them and fitting others to experimental data from spectroscopy. Methodology Quantum mechanics provides equations based on the Hartree–Fock method and the Roothaan equations that CNDO uses to model atoms and their locations. These equations are solved iterat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |