|
Etilamphetamine
Etilamfetamine (trade names Apetinil and Adiparthrol; also known as ''N''-ethylamphetamine) is a stimulant drug of the phenethylamine and amphetamine chemical classes. It was invented in the early 20th century and was subsequently used as an anorectic or appetite suppressant in the 1950s, but was not as commonly used as other amphetamines such as amphetamine, methamphetamine, and benzphetamine, and was largely discontinued once newer drugs such as phenmetrazine were introduced. It most likely acts primarily as a dopamine releasing agent. Its activity as a norepinephrine or serotonin releasing agent is not known. Chemistry The molecular structure of ethylamphetamine is analogous to methamphetamine, with an ethyl group in place of the methyl group.Amphetamine is a substituted phenethylamine with a methyl group at RA position. It can also be considered a substituted amphetamine, with an ethyl group on the amphetamine backbone.The ethyl group of ethylamphetamine is at RN po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mouth
In animal anatomy, the mouth, also known as the oral cavity, or in Latin cavum oris, is the opening through which many animals take in food and issue vocal sounds. It is also the cavity lying at the upper end of the alimentary canal, bounded on the outside by the lips and inside by the pharynx. In tetrapods, it contains the tongue and, except for some like birds, teeth. This cavity is also known as the buccal cavity, from the Latin ''bucca'' ("cheek"). Some animal phyla, including arthropods, molluscs and chordates, have a complete digestive system, with a mouth at one end and an anus at the other. Which end forms first in ontogeny is a criterion used to classify bilaterian animals into protostomes and deuterostomes. Development In the first multicellular animals, there was probably no mouth or gut and food particles were engulfed by the cells on the exterior surface by a process known as endocytosis. The particles became enclosed in vacuoles into which enzymes were secr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzphetamine
Benzphetamine (brand name Didrex) is a substituted amphetamine used short-term along with a doctor-approved, reduced- calorie diet, exercise, and behavioral program for weight loss. It is prescribed for obesity to people who have been unable to lose weight through exercise and dieting alone. It is a prodrug to dextroamphetamine and dextromethamphetamine. Benzphetamine is an anorectic, primarily promoting weight loss through reduced appetite. It also slightly increases metabolism. Pharmacology Benzphetamine is a sympathomimetic amine and is classified as an anorectic. The drug's main function is to reduce appetite, which in turn reduces caloric intake. Although the mechanism of action of the sympathomimetic appetite suppressants in the treatment of obesity is not fully known, these medications have pharmacological effects similar to those of amphetamines. Amphetamine and related sympathomimetic medications (such as benzphetamine) are thought to stimulate the release of nor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituted Amphetamines
Substituted amphetamines are a class of compounds based upon the amphetamine structure; it includes all derivative compounds which are formed by replacing, or substituting, one or more hydrogen atoms in the amphetamine core structure with substituents. The compounds in this class span a variety of pharmacological subclasses, including stimulants, empathogens, and hallucinogens, among others. Examples of substituted amphetamines are amphetamine (itself), methamphetamine, ephedrine, cathinone, phentermine, mephentermine, bupropion, methoxyphenamine, selegiline, amfepramone (diethylpropion), pyrovalerone, MDMA (ecstasy), and DOM (STP). Some of amphetamine's substituted derivatives occur in nature, for example in the leaves of ''Ephedra'' and khat plants. Amphetamine was first produced at the end of the 19th century. By the 1930s, amphetamine and some of its derivative compounds found use as decongestants in the symptomatic treatment of colds and also occasionally as psychoac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propylamphetamine
Propylamphetamine is a psychoactive drug and research chemical of the phenethylamine and amphetamine chemical classes which acts as a stimulant. It was first developed in the 1970s, mainly for research into the metabolism of, and as a comparison tool to, other amphetamines. A study in rats found propylamphetamine to be 1/4 as potent as amphetamine. See also * Amphetamine * Ethylamphetamine Etilamfetamine (trade names Apetinil and Adiparthrol; also known as ''N''-ethylamphetamine) is a stimulant drug of the phenethylamine and amphetamine chemical classes. It was invented in the early 20th century and was subsequently used as an ano ... * Isopropylamphetamine * Methamphetamine * Phenylpropylaminopentane References Substituted amphetamines Norepinephrine-dopamine releasing agents {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isopropylamphetamine
Isopropylamphetamine is a psychostimulant of the substituted amphetamine class. It is an isomer of propylamphetamine and was discovered by a team at Astra Laekemedel AB. The isopropyl moiety reduces the stimulant activity of the compound but greatly increases the duration of action. For this reason, the compound is not used recreationally. See also * Amphetamine * Ethylamphetamine * Isoprenaline * Methamphetamine * Propylamphetamine Propylamphetamine is a psychoactive drug and research chemical of the phenethylamine and amphetamine chemical classes which acts as a stimulant. It was first developed in the 1970s, mainly for research into the metabolism of, and as a compa ... References Stimulants Substituted amphetamines Norepinephrine-dopamine releasing agents Isopropyl compounds Secondary amines {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fenfluramine
Fenfluramine, sold under the brand name Fintepla, is a serotonergic medication used for the treatment of seizures associated with Dravet syndrome and Lennox–Gastaut syndrome.https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/212102s003lbl.pdf It was formerly used as an appetite suppressant in the treatment of obesity, but was discontinued for this use due to cardiovascular toxicity before being repurposed for new indications. Fenfluramine was used for weight loss both alone under the brand name Pondimin and in combination with phentermine under the brand name Fen-Phen among others. Side effects of fenfluramine in people treated for seizures include decreased appetite, somnolence, sedation, lethargy, diarrhea, constipation, abnormal echocardiogram, fatigue, malaise, asthenia, ataxia, balance disorder, gait disturbance, increased blood pressure, drooling, excessive salivation, fever, upper respiratory tract infection, vomiting, appetite loss, weight loss, falls, and s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylamphetamine
Dimethylamphetamine (Metrotonin), also known as dimetamfetamine (INN), dimephenopan and ''N'',''N''-dimethylamphetamine, is a stimulant drug of the phenethylamine and amphetamine chemical class Chemical classification systems attempt to classify elements or compounds according to certain chemical functional or structural properties. Whereas the structural properties are largely intrinsic, functional properties and the derived classificat ...es. Dimethylamphetamine has weaker stimulant effects than amphetamine or methamphetamine and is considerably less addictive and less neurotoxic compared to methamphetamine. However, it still retains some mild stimulant effects and abuse potential, and is a Schedule I controlled drug. Dimethylamphetamine has occasionally been found in illicit methamphetamine laboratories, but is usually an impurity rather than the desired product. It may be produced by accident when methamphetamine is synthesised by methylation of amphetamine if the re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-Fluoroethamphetamine
3-Fluoroethamphetamine (3-FEA) is a stimulant drug of the amphetamine class which acts as a releasing agent of the monoamine neurotransmitters norepinephrine, dopamine and serotonin. Compared to the unsubstituted ethylamphetamine, 3-fluoroethamphetamine is a weaker releaser of noradrenaline, but a stronger releaser of both dopamine and serotonin, and produced the strongest reinforcing effects in animal studies out of a range of 3-substituted ethamphetamine derivatives tested, despite not being the most potent dopamine releaser. See also * 2-Fluoroamphetamine * 2-Fluoromethamphetamine * 3-Fluoroamphetamine * 3-Fluoromethamphetamine * 4-Fluoroamphetamine * 4-Fluoromethamphetamine * Fenfluramine Fenfluramine, sold under the brand name Fintepla, is a serotonergic medication used for the treatment of seizures associated with Dravet syndrome and Lennox–Gastaut syndrome.https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/212102s003lb ... References {{DEFAULTS ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
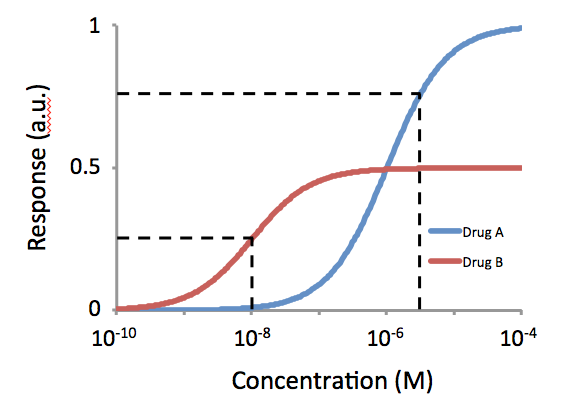
Potency (pharmacology)
In the field of pharmacology, potency is a measure of drug activity expressed in terms of the amount required to produce an effect of given intensity. A highly potent drug (e.g., fentanyl, alprazolam, risperidone, bumetanide, bisoprolol) evokes a given response at low concentrations, while a drug of lower potency (meperidine, diazepam, ziprasidone, furosemide, metoprolol) evokes the same response only at higher concentrations. Higher potency does not necessarily mean greater effectiveness or more side effects. The IUPHAR The International Union of Basic and Clinical Pharmacology (IUPHAR) is a voluntary, non-profit association representing the interests of scientists in pharmacology-related fields to facilitate ''Better Medicines through Global Education and Resear ... has stated that 'potency' is ''"an imprecise term that should always be further defined"'', for instance as EC_, IC_, ED_, LD_ and so on. See also * Reaction inhibitor § Potency References Further readin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Recreational Drug
Recreational drug use indicates the use of one or more psychoactive drugs to induce an altered state of consciousness either for pleasure or for some other casual purpose or pastime by modifying the perceptions and emotions of the user. When a psychoactive drug enters the user's body, it induces an intoxicating effect. Generally, recreational drugs are divided into three categories: depressants (drugs that induce a feeling of relaxation and calmness); stimulants (drugs that induce a sense of energy and alertness); and hallucinogens (drugs that induce perceptual distortions such as hallucination). In popular practice, recreational drug use generally is a tolerated social behaviour, rather than perceived as the medical condition of self-medication. However, heavy use of some drugs is socially stigmatized. Many people also use prescribed and controlled depressants such as opioids, as well as opiates and benzodiazepines. Common recreational drugs include caffeine, commonly foun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Group
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bounded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources of the cation, and this simplification is used pervasively in organic chemistry. For example, protonation of methanol gives an elect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Group
In organic chemistry, an ethyl group (abbr. Et) is an alkyl substituent with the formula , derived from ethane (). ''Ethyl'' is used in the International Union of Pure and Applied Chemistry's nomenclature of organic chemistry for a saturated two-carbon moiety in a molecule, while the prefix "''eth-''" is used to indicate the presence of two carbon atoms in the molecule. Ethylation Ethylation is the formation of a compound by introduction of the ethyl group. The most widely practiced example of this reaction is the ethylation of benzene with ethylene to yield ethylbenzene, a precursor to styrene, which is a precursor to polystyrene. Approximately 24.7 million tons of ethylbenzene were produced in 1999. :: Many ethyl-containing compounds are generated by electrophilic ethylation, i.e. treatment of nucleophiles with sources of Et+. Triethyloxonium tetrafluoroborate t3OF4 is such a reagent. For good nucleophiles, less electrophilic reagents are employed, such as ethyl h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |