|
Ethylmercury
Ethylmercury (sometimes ethyl mercury) is a cation composed of an organic CH3CH2- species (an ethyl group) bound to a mercury(II) centre, making it a type of organometallic cation, and giving it a chemical formula C2H5Hg+. The main source of ethylmercury is thimerosal. Synthesis and structure Ethylmercury (C2H5Hg+) is a substituent of compounds: it occurs as a component of compounds of the formula C2H5HgX where X = chloride, thiolate, or another organic group. Most famously X = the mercaptide group of thiosalicylic acid as in thiomersal. In the body, ethylmercury is most commonly encountered as derivatives with a thiolate attached to the mercury. In these compounds, Hg(II) has a linear or sometimes trigonal coordination geometry. Given the comparable electronegativities of mercury and carbon, the mercury-carbon bond is described as covalent. Toxicity The toxicity of ethylmercury is well studied. Like methylmercury, ethylmercury distributes to all body tissues, crossing t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiomersal
Thiomersal (INN), or thimerosal (USAN, JAN), is an organomercury compound. It is a well-established antiseptic and antifungal agent. The pharmaceutical corporation Eli Lilly and Company gave thiomersal the trade name Merthiolate. It has been used as a preservative in vaccines, immunoglobulin preparations, skin test antigens, antivenins, ophthalmic and nasal products, and tattoo inks. In spite of the scientific consensus that fears about its safety are unsubstantiated, its use as a vaccine preservative has been called into question by anti-vaccination groups. Due to this public pressure the substance was phased out of routine childhood vaccines in the United States, the European Union, and a few other countries in response to popular fears. It remains in use as a preservative for annual flu vaccines. History Morris Kharasch, a chemist then at the University of Maryland filed a patent application for thiomersal in 1927; Eli Lilly later marketed the compound under the trade ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thimerosal
Thiomersal (INN), or thimerosal (USAN, JAN), is an organomercury compound. It is a well-established antiseptic and antifungal agent. The pharmaceutical corporation Eli Lilly and Company gave thiomersal the trade name Merthiolate. It has been used as a preservative in vaccines, immunoglobulin preparations, skin test antigens, antivenins, ophthalmic and nasal products, and tattoo inks. In spite of the scientific consensus that fears about its safety are unsubstantiated, its use as a vaccine preservative has been called into question by anti-vaccination groups. Due to this public pressure the substance was phased out of routine childhood vaccines in the United States, the European Union, and a few other countries in response to popular fears. It remains in use as a preservative for annual flu vaccines. History Morris Kharasch, a chemist then at the University of Maryland filed a patent application for thiomersal in 1927; Eli Lilly later marketed the compound under the trade ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury Poisoning
Mercury poisoning is a type of metal poisoning due to exposure to mercury. Symptoms depend upon the type, dose, method, and duration of exposure. They may include muscle weakness, poor coordination, numbness in the hands and feet, skin rashes, anxiety, memory problems, trouble speaking, trouble hearing, or trouble seeing. High-level exposure to methylmercury is known as Minamata disease. Methylmercury exposure in children may result in acrodynia (pink disease) in which the skin becomes pink and peels. Long-term complications may include kidney problems and decreased intelligence. The effects of long-term low-dose exposure to methylmercury are unclear. Forms of mercury exposure include metal, vapor, salt, and organic compound. Most exposure is from eating fish, amalgam-based dental fillings, or exposure at a workplace. In fish, those higher up in the food chain generally have higher levels of mercury, a process known as biomagnification. Less commonly, poisoning may occur a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethylmercury
Diethylmercury is a flammable, colorless liquid, and one of the strongest known neurotoxins. This organomercury compound is described as having a slightly sweet smell, though inhaling enough fumes to notice this would be hazardous. This chemical can cross the blood–brain barrier, causing permanent brain damage. It is, however, considerably less toxic than dimethylmercury. Synthesis Diethylmercury can be obtained from the reaction between ethylmagnesium bromide and mercury(II) chloride. :2 C2H5MgBr + HgCl2 → Hg(C2H5)2 + MgBr2 + MgCl2 Other methods are also known. See also * Dimethylmercury, a related compound * Ethylmercury * Mercury poisoning Mercury poisoning is a type of metal poisoning due to exposure to mercury. Symptoms depend upon the type, dose, method, and duration of exposure. They may include muscle weakness, poor coordination, numbness in the hands and feet, skin rashe ... References {{DEFAULTSORT:Diethyl Mercury Organomercury compounds Sweet-sme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylmercury
Methylmercury (sometimes methyl mercury) is an organometallic cation with the formula . It is the simplest organomercury compound. Methylmercury is extremely toxic, and its derivatives are the major source of organic mercury for humans. It is a bioaccumulative environmental toxicant. Structure and chemistry "Methylmercury" is a shorthand for the hypothetical "methylmercury cation", sometimes written "''methylmercury(1+) cation''" or "''methylmercury(II) cation''". This functional group is composed of a methyl group bonded to an atom of mercury. Its chemical formula is (sometimes written as ).The Methylmercury compound has an overall charge of +1, with Hg in the +2 oxidation state.Methylmercury exists as a substituent in many complexes of the type (L = Lewis base) and MeHgX (X = anion). As a positively charged ion it readily combines with anions such as chloride (), hydroxide () and nitrate (). It has particular affinity for sulfur-containing anions, particularly thiols (). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blood–brain Barrier
The blood–brain barrier (BBB) is a highly selective semipermeable membrane, semipermeable border of endothelium, endothelial cells that prevents solutes in the circulating blood from ''non-selectively'' crossing into the extracellular fluid of the central nervous system where neurons reside. The blood–brain barrier is formed by endothelial cells of the Capillary, capillary wall, astrocyte end-feet ensheathing the capillary, and pericytes embedded in the capillary basement membrane. This system allows the passage of some small molecules by passive transport, passive diffusion, as well as the selective and active transport of various nutrients, ions, organic anions, and macromolecules such as glucose and amino acids that are crucial to neural function. The blood–brain barrier restricts the passage of pathogens, the diffusion of solutes in the blood, and Molecular mass, large or Hydrophile, hydrophilic molecules into the cerebrospinal fluid, while allowing the diffusion of Hydr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organomercury Compounds
Organomercury refers to the group of organometallic compounds that contain mercury. Typically the Hg–C bond is stable toward air and moisture but sensitive to light. Important organomercury compounds are the methylmercury(II) cation, CH3Hg+; ethylmercury(II) cation, C2H5Hg+; dimethylmercury, (CH3)2Hg, diethylmercury and merbromin ("Mercurochrome"). Thiomersal is used as a preservative for vaccines and intravenous drugs. The toxicity of organomercury compounds presents both dangers and benefits. Dimethylmercury in particular, is notoriously toxic, but found use as an antifungal agent and insecticide. Merbromin and phenylmercuric borate are used as topical antiseptics, while nitromersol is used as a preservative for vaccines and antitoxins. Synthesis Organomercury compounds are generated by many methods, including the direct reaction of hydrocarbons and mercury(II) salts. In this regard, organomercury chemistry more closely resembles organopalladium chemistry and contrasts wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable atoms survive. The term is also used more generally to characterize any type of exponential (or, rarely, non-exponential) decay. For example, the medical sciences refer to the biological half-life of drugs and other chemicals in the human body. The converse of half-life (in exponential growth) is doubling time. The original term, ''half-life period'', dating to Ernest Rutherford's discovery of the principle in 1907, was shortened to ''half-life'' in the early 1950s. Rutherford applied the principle of a radioactive element's half-life in studies of age determination of rocks by measuring the decay period of radium to lead-206. Half-life is constant over the lifetime of an exponentially decaying quantity, and it is a characteristic unit for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
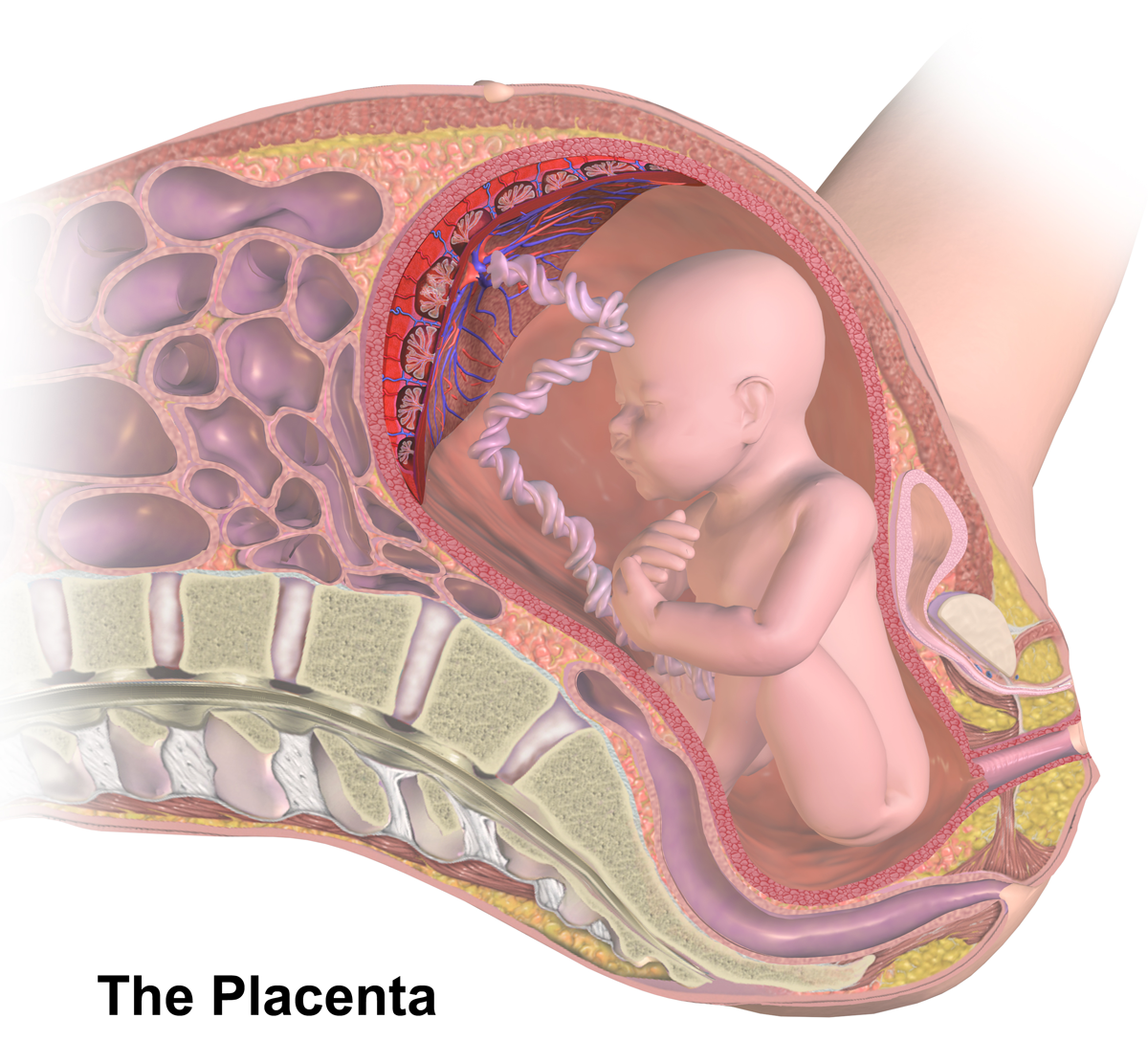
Placental Barrier
The placenta is a temporary embryonic and later fetal organ that begins developing from the blastocyst shortly after implantation. It plays critical roles in facilitating nutrient, gas and waste exchange between the physically separate maternal and fetal circulations, and is an important endocrine organ, producing hormones that regulate both maternal and fetal physiology during pregnancy. The placenta connects to the fetus via the umbilical cord, and on the opposite aspect to the maternal uterus in a species-dependent manner. In humans, a thin layer of maternal decidual (endometrial) tissue comes away with the placenta when it is expelled from the uterus following birth (sometimes incorrectly referred to as the 'maternal part' of the placenta). Placentas are a defining characteristic of placental mammals, but are also found in marsupials and some non-mammals with varying levels of development. Mammalian placentas probably first evolved about 150 million to 200 million years ag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity, the more an atom or a substituent group attracts electrons. Electronegativity serves as a simple way to quantitatively estimate the bond energy, and the sign and magnitude of a bond's chemical polarity, which characterizes a bond along the continuous scale from covalent to ionic bonding. The loosely defined term electropositivity is the opposite of electronegativity: it characterizes an element's tendency to donate valence electrons. On the most basic level, electronegativity is determined by factors like the nuclear charge (the more protons an atom has, the more "pull" it will have on electrons) and the number and location ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical ( in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (included in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)