|
Equilenin
Equilenin, also known as 6,8-didehydroestrone, as well as estra-1,3,5(10),6,8-pentaen-3-ol-17-one, is a naturally occurring steroidal estrogen obtained from the urine of pregnant mares. It is used as one of the components in conjugated estrogens (brand name Premarin). It was the first complex natural product to be fully synthesized, in work reported by 1940 by Bachmann and Wilds. Chemistry Synthesis Total synthesis The synthesis developed by the Bachmann group started from Butenand's ketone – the 7-methoxy structural analog of 1,2,3,4-tetrahydrophenanthren-1-one – and which can be readily prepared from 1,6- Cleve's acid. The approach was based on well-established transformations like the Claisen condensation, the Reformatsky reaction, the Arndt–Eistert reaction, and the Dieckmann condensation. Nicolaou described this preparation as ending the era preceding the post-World War II work of Robert Burns Woodward Robert Burns Woodward (April 10, 1917 – July ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Estrogens ...
This is a list of steroidal estrogens or derivatives of estradiol, estrone, and estriol. Most esters of these estrogens are not included in this list; for esters, see here instead. Estradiol derivatives 17α-Substituted estradiol derivatives Nitrogen mustard-coupled alkylating antineoplastic estradiol derivatives 17β-Aminoestrogens Estrone derivatives Nitrogen mustard-coupled alkylating antineoplastic estrone derivatives Estriol derivatives 17α-Substituted estriol derivatives Other estrogen derivatives Epimers Equine estrogens See also * List of steroids Notes ? = Chemical names that are unverified. References {{Estrogen receptor modulators Estrogens Steroids Estrogens Estrogen or oestrogen is a category of sex hormone responsible for the development and regulation of the female reproductive system and secondary sex characteristics. There are three major endogenous estrogens that have estrogenic hormonal acti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Werner Emmanuel Bachmann
Werner Emmanuel Bachmann (November 13, 1901 – March 22, 1951) was an American chemist. Bachmann was born in Detroit, Michigan where he studied chemistry and chemical engineering at Wayne State University and later at the University of Michigan in Ann Arbor nearby. He completed his doctorate under Moses Gomberg and spent the rest of his academic career at the University of Michigan. Bachmann studied physical organic chemistry (rearrangements, free radicals) and organic synthesis. He is considered a pioneer in steroid synthesis, and carried out the first total synthesis of a steroidal hormone, equilenin with Alfred L. Wilds. His name is associated with the Gomberg-Bachmann reaction for the synthesis of diaryl compounds from aryl diazonium chlorides. Bachmann developed a new method for the production of the explosive RDX, which was used by the United States during World War II World War II or the Second World War, often abbreviated as WWII or WW2, was a world ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oral Administration
Oral administration is a route of administration where a substance is taken through the mouth. Per os abbreviated to P.O. is sometimes used as a direction for medication to be taken orally. Many medications are taken orally because they are intended to have a systemic effect, reaching different parts of the body via the bloodstream, for example. Oral administration can be easier and less painful than other routes, such as injection. However, the onset of action is relatively low, and the effectiveness is reduced if it is not absorbed properly in the digestive system, or if it is broken down by digestive enzymes before it can reach the bloodstream. Some medications may cause gastrointestinal side effects, such as nausea or vomiting, when taken orally. Oral administration can also only be applied to conscious patients, and patients willing and able to swallow. Terminology ''Per os'' (; ''P.O.'') is an adverbial phrase meaning literally from Latin "through the mouth" or "by mouth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Claisen Condensation
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base, resulting in a β-keto ester or a β-diketone. It is named after Rainer Ludwig Claisen, who first published his work on the reaction in 1887. Requirements At least one of the reagents must be enolizable (have an α-proton and be able to undergo deprotonation to form the enolate anion). There are a number of different combinations of enolizable and nonenolizable carbonyl compounds that form a few different types of Claisen. The base used must not interfere with the reaction by undergoing nucleophilic substitution or addition with a carbonyl carbon. For this reason, the conjugate sodium alkoxide base of the alcohol formed (e.g. sodium ethoxide if ethanol is formed) is often used, since the alkoxide is regenerated. In mixed Claisen condensations, a non-nucleophilic base such as lithium diisopropylamide, or L ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Estranes
Estrane is a C18 steroid derivative, with a gonane core. ''Estrenes'' are estrane derivatives that contain a double bond, with an example being nandrolone. ''Estratrienes'' (estrins) are estrane derivatives that contain three double bonds, for instance estrin (estra-1,3,5(10)-triene). The estrogen steroid hormones estradiol, estrone, and estriol are estra-1,3,5(10)-trienes. See also * Androstane * Pregnane Pregnane, also known as 17β-ethylandrostane or as 10β,13β-dimethyl-17β-ethylgonane, is a C21 steroid and, indirectly, a parent of progesterone. It is a parent hydrocarbon for two series of steroids stemming from 5α-pregnane (originally allop ... References Estranes {{Steroid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (— O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule. Phenols are both synthesized industrially and produced by plants and microorganisms. Properties Acidity Phenols are more acidic than typical alcohols. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12). Deprotonation of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides (aryloxides according to the IUPAC Gold Book). Condensation with aldehydes and ketones Phenols are susceptible to Electrophilic aromatic substitutions. Condensation with formald ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sterols
Sterol is an organic compound with formula , whose molecule is derived from that of gonane by replacement of a hydrogen atom in position 3 by a hydroxyl group. It is therefore an alcohol of gonane. More generally, any compounds that contain the gonane structure, additional functional groups, and/or modified ring systems derived from gonane are called steroids. Therefore, sterols are a subgroup of the steroids. They occur naturally in most eukaryotes, including plants, animals, and fungi, and can also be produced by some bacteria (however likely with different functions). The most familiar type of animal sterol is cholesterol, which is vital to cell membrane structure, and functions as a precursor to fat-soluble vitamins and steroid hormones. While technically alcohols, sterols are classified by biochemists as lipids (fats in the broader sense of the term). Types Sterols of plants are called ''phytosterols'' and sterols of animals are called ''zoosterols''. The most important ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chiral Resolution
Chiral resolution, or enantiomeric resolution, is a process in stereochemistry for the separation of racemic compounds into their enantiomers. It is an important tool in the production of optically active compounds, including drugs. Another term with the same meaning is optical resolution. The use of chiral resolution to obtain enantiomerically pure compounds has the disadvantage of necessarily discarding at least half of the starting racemic mixture. Asymmetric synthesis of one of the enantiomers is one means of avoiding this waste. Crystallization of diastereomeric salts The most common method for chiral resolution involves conversion of the racemic mixture to a pair of diastereomeric derivatives by reacting them with chiral derivatizing agents, also known as chiral resolving agents. The derivatives which are then separated by conventional crystallization, and converted back to the enantiomers by removal of the resolving agent. The process can be laborious and depends on the div ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enantioselective Synthesis
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric (enantiomeric or diastereomeric) products in unequal amounts." Put more simply: it is the synthesis of a compound by a method that favors the formation of a specific enantiomer or diastereomer. Enantiomers are stereoisomers that have opposite configurations at every chiral center. Diastereomers are stereoisomers that differ at one or more chiral centers. Enantioselective synthesis is a key process in modern chemistry and is particularly important in the field of pharmaceuticals, as the different enantiomers or diastereomers of a molecule often have different biological activity. Overview Many of the building blocks of biological systems such as sugars and amino acids are produced exclusively as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Robert Burns Woodward
Robert Burns Woodward (April 10, 1917 – July 8, 1979) was an American organic chemist. He is considered by many to be the most preeminent synthetic organic chemist of the twentieth century, having made many key contributions to the subject, especially in the synthesis of complex natural products and the determination of their molecular structure. He also worked closely with Roald Hoffmann on theoretical studies of chemical reactions. He was awarded the Nobel Prize in Chemistry in 1965. Early life and education Woodward was born in Boston, Massachusetts, on April 10, 1917. He was the son of Margaret Burns (an immigrant from Scotland who claimed to be a descendant of the poet, Robert Burns) and her husband, Arthur Chester Woodward, himself the son of Roxbury apothecary, Harlow Elliot Woodward. His father was one of the many victims of the 1918 influenza pandemic of 1918. From a very early age, Woodward was attracted to and engaged in private study of chemistry while he att ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
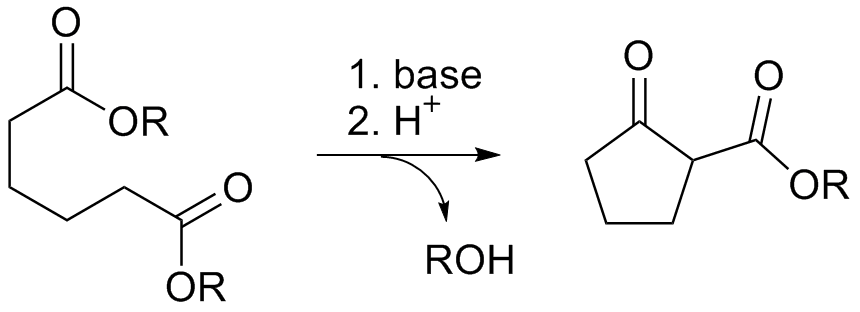
Dieckmann Condensation
The Dieckmann condensation is the intramolecular chemical reaction of diesters with base to give β-keto esters. It is named after the German chemist Walter Dieckmann (1869–1925). The equivalent intermolecular reaction is the Claisen condensation. : Reaction mechanism Deprotonation of an ester at the α-position generates an enolate ion which then undergoes a 5-exo-trig nucleophilic attack to give a cyclic enol. Protonation with a Brønsted-Lowry acid (H3O+ for example) re-forms the β-keto ester. : Due to the steric stability of five- and six-membered rings, these structures will preferentially be formed. 1,6 diesters will form five-membered cyclic β-keto esters, while 1,7 diesters will form six-membered β-keto esters. Further reading *Dieckmann, W. '' Ber.'' 1894, ''27'', 102 & 965 *Dieckmann, W. ''Ber.'' 1900, ''33'', 595 & 2670 *Dieckmann, W. ''Ann.'' 1901, ''317'', 51 & 93 See also * Claisen condensation * Gabriel-Colman rearrangement * Thorpe–Ziegler reaction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |