|
Epimestrol
Epimestrol (, , ) (brand names Alene, Stimovul; former developmental code name ORG-817), also known as 3-methoxy-17-epiestriol, is a synthetic, steroidal estrogen and an estrogen ether and prodrug of 17-epiestriol. It has been used as a component of ovulation induction in combination with gonadotropin-releasing hormone. See also * List of estrogens This is a list of steroidal estrogens or derivatives of estradiol, estrone, and estriol. Most esters of these estrogens are not included in this list; for esters, see here instead. Estradiol derivatives 17α-Substituted estradiol derivative ... References Estranes Ethers Synthetic estrogens Triols {{Steroid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Estrogens ...
This is a list of steroidal estrogens or derivatives of estradiol, estrone, and estriol. Most esters of these estrogens are not included in this list; for esters, see here instead. Estradiol derivatives 17α-Substituted estradiol derivatives Nitrogen mustard-coupled alkylating antineoplastic estradiol derivatives 17β-Aminoestrogens Estrone derivatives Nitrogen mustard-coupled alkylating antineoplastic estrone derivatives Estriol derivatives 17α-Substituted estriol derivatives Other estrogen derivatives Epimers Equine estrogens See also * List of steroids Notes ? = Chemical names that are unverified. References {{Estrogen receptor modulators Estrogens Steroids Estrogens Estrogen or oestrogen is a category of sex hormone responsible for the development and regulation of the female reproductive system and secondary sex characteristics. There are three major endogenous estrogens that have estrogenic hormonal acti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
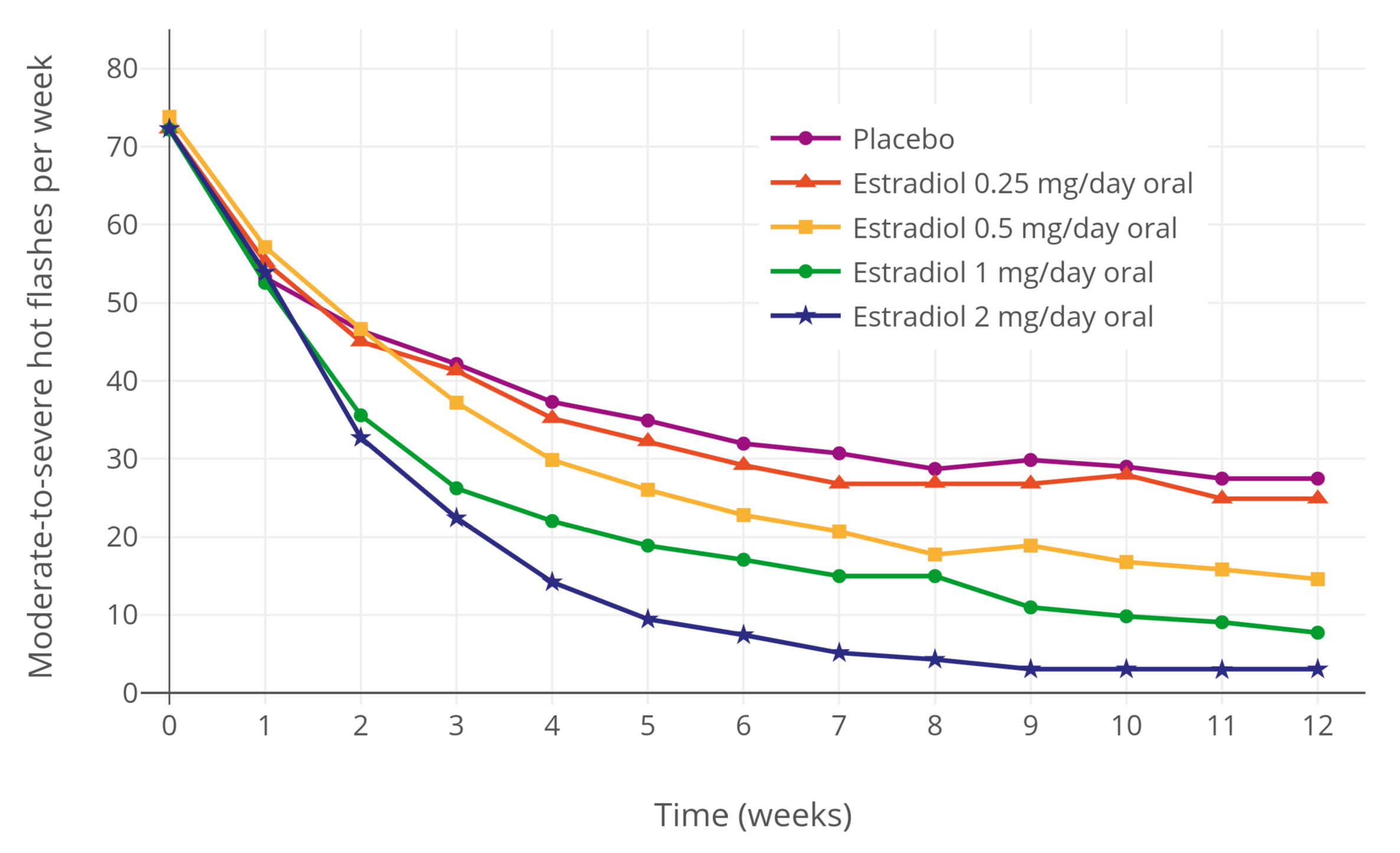
Oral Administration
Oral administration is a route of administration where a substance is taken through the mouth. Per os abbreviated to P.O. is sometimes used as a direction for medication to be taken orally. Many medications are taken orally because they are intended to have a systemic effect, reaching different parts of the body via the bloodstream, for example. Oral administration can be easier and less painful than other routes, such as injection. However, the onset of action is relatively low, and the effectiveness is reduced if it is not absorbed properly in the digestive system, or if it is broken down by digestive enzymes before it can reach the bloodstream. Some medications may cause gastrointestinal side effects, such as nausea or vomiting, when taken orally. Oral administration can also only be applied to conscious patients, and patients willing and able to swallow. Terminology ''Per os'' (; ''P.O.'') is an adverbial phrase meaning literally from Latin "through the mouth" or "by mouth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Estrogen (medication)
An estrogen (E) is a type of medication which is used most commonly in hormonal birth control and menopausal hormone therapy, and as part of feminizing hormone therapy for transgender women. They can also be used in the treatment of hormone-sensitive cancers like breast cancer and prostate cancer and for various other indications. Estrogens are used alone or in combination with progestogens. They are available in a wide variety of formulations and for use by many different routes of administration. Examples of estrogens include bioidentical estradiol, natural conjugated estrogens, synthetic steroidal estrogens like ethinylestradiol, and synthetic nonsteroidal estrogens like diethylstilbestrol. Estrogens are one of three types of sex hormone agonists, the others being androgens/anabolic steroids like testosterone and progestogens like progesterone. Side effects of estrogens include breast tenderness, breast enlargement, headache, nausea, fluid retention, and edema among other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Estrogen Ether
An estrogen ester is an ester of an estrogen, most typically of estradiol but also of other estrogens such as estrone, estriol, and even nonsteroidal estrogens like diethylstilbestrol. Esterification renders estradiol into a prodrug of estradiol with increased resistance to first-pass metabolism, slightly improving its oral bioavailability. In addition, estrogen esters have increased lipophilicity, which results in a longer duration when given by intramuscular or subcutaneous injection due to the formation of a long-lasting local depot in muscle and fat. Conversely, this is not the case with intravenous injection or oral administration. Estrogen esters are rapidly hydrolyzed into their parent estrogen by esterases once they have been released from the depot. Because estradiol esters are prodrugs of estradiol, they are considered to be natural and bioidentical forms of estrogen. Estrogen esters are used in hormone therapy, hormonal contraception, and high-dose estrogen therapy (e.g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthetic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Living t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steroid
A steroid is a biologically active organic compound with four rings arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and as signaling molecules. Hundreds of steroids are found in plants, animals and fungi. All steroids are manufactured in cells from the sterols lanosterol (opisthokonts) or cycloartenol (plants). Lanosterol and cycloartenol are derived from the cyclization of the triterpene squalene. The steroid core structure is typically composed of seventeen carbon atoms, bonded in four " fused" rings: three six-member cyclohexane rings (rings A, B and C in the first illustration) and one five-member cyclopentane ring (the D ring). Steroids vary by the functional groups attached to this four-ring core and by the oxidation state of the rings. Sterols are forms of steroids with a hydroxy group at position three and a skeleton derived from cholestane. ''A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Estrogen (medication)
An estrogen (E) is a type of medication which is used most commonly in hormonal birth control and menopausal hormone therapy, and as part of feminizing hormone therapy for transgender women. They can also be used in the treatment of hormone-sensitive cancers like breast cancer and prostate cancer and for various other indications. Estrogens are used alone or in combination with progestogens. They are available in a wide variety of formulations and for use by many different routes of administration. Examples of estrogens include bioidentical estradiol, natural conjugated estrogens, synthetic steroidal estrogens like ethinylestradiol, and synthetic nonsteroidal estrogens like diethylstilbestrol. Estrogens are one of three types of sex hormone agonists, the others being androgens/anabolic steroids like testosterone and progestogens like progesterone. Side effects of estrogens include breast tenderness, breast enlargement, headache, nausea, fluid retention, and edema among other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Estrogen Ether
An estrogen ester is an ester of an estrogen, most typically of estradiol but also of other estrogens such as estrone, estriol, and even nonsteroidal estrogens like diethylstilbestrol. Esterification renders estradiol into a prodrug of estradiol with increased resistance to first-pass metabolism, slightly improving its oral bioavailability. In addition, estrogen esters have increased lipophilicity, which results in a longer duration when given by intramuscular or subcutaneous injection due to the formation of a long-lasting local depot in muscle and fat. Conversely, this is not the case with intravenous injection or oral administration. Estrogen esters are rapidly hydrolyzed into their parent estrogen by esterases once they have been released from the depot. Because estradiol esters are prodrugs of estradiol, they are considered to be natural and bioidentical forms of estrogen. Estrogen esters are used in hormone therapy, hormonal contraception, and high-dose estrogen therapy (e.g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prodrug
A prodrug is a medication or compound that, after intake, is metabolized (i.e., converted within the body) into a pharmacologically active drug. Instead of administering a drug directly, a corresponding prodrug can be used to improve how the drug is absorbed, distributed, metabolized, and excreted (ADME). Prodrugs are often designed to improve bioavailability when a drug itself is poorly absorbed from the gastrointestinal tract. A prodrug may be used to improve how selectively the drug interacts with cells or processes that are not its intended target. This reduces adverse or unintended effects of a drug, especially important in treatments like chemotherapy, which can have severe unintended and undesirable side effects. History Many herbal extracts historically used in medicine contain glycosides (sugar derivatives) of the active agent, which are hydrolyzed in the intestines to release the active and more bioavailable aglycone. For example, salicin is a β-D-glucopyranosid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ovulation Induction
Ovulation induction is the stimulation of ovulation by medication. It is usually used in the sense of stimulation of the development of ovarian follicles Ovulation Induction Retrieved on Mars 7, 2010 to reverse anovulation or oligoovulation. Scope The term ''ovulation induction'' can potentially also be used for: *Final maturation induction, in the sense of triggering ''oocyte release'' from relatively mature ovarian follicles during late follicular phase. In any case, ovarian stimulation (in the sense of stimulating the development of oocytes) is often used in conjunction with triggering oocyte release, such as for proper timing of artificial insemination. *Controlled ovarian hyperstimulation (stimulating the development of multiple follicles of the ovaries in one single cycle), has also appeared in the scope of ovulation induction.IVF.com > Ovulation Induction Retrieved on Mars 7, 2010 Controlled ovarian hyperstimulation is generally part of in vitro fertilization, and the aim i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gonadotropin-releasing Hormone
Gonadotropin-releasing hormone (GnRH) is a releasing hormone responsible for the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary. GnRH is a tropic peptide hormone synthesized and released from GnRH neurons within the hypothalamus. The peptide belongs to gonadotropin-releasing hormone family. It constitutes the initial step in the hypothalamic–pituitary–gonadal axis. Structure The identity of GnRH was clarified by the 1977 Nobel Laureates Roger Guillemin and Andrew V. Schally: pyroGlu-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly-NH2 As is standard for peptide representation, the sequence is given from amino terminus to carboxyl terminus; also standard is omission of the designation of chirality, with assumption that all amino acids are in their L- form. The abbreviations are the standard abbreviations for the corresponding proteinogenic amino acids, except for ''pyroGlu'', which refers to pyroglutamic acid, a derivative of g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |