|
Electroplating
Electroplating, also known as electrochemical deposition or electrodeposition, is a process for producing a metal coating on a solid substrate through the redox, reduction of cations of that metal by means of a direct current, direct electric current. The part to be coated acts as the cathode (negative electrode) of an electrolytic cell; the electrolyte is a solution (chemistry), solution of a salt (chemistry), salt whose cation is the metal to be coated, and the anode (positive electrode) is usually either a block of that metal, or of some inert electrical conductor, conductive material. The current is provided by an external power supply. Electroplating is widely used in industry and decorative arts to improve the surface qualities of objects—such as resistance to abrasion (mechanical), abrasion and corrosion, lubrication, lubricity, reflectivity, electrical conductivity, or appearance. It is used to build up thickness on undersized or worn-out parts and to manufacture metal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional current describes the direction in which positive charges move. Electrons, which are the carriers of current in most electrical systems, have a negative electrical charge, so the movement of electrons is ''opposite'' to that of the conventional current flow: this means that electrons flow ''into'' the device's cathode from the external circuit. For example, the end of a household battery marked with a + (plus) is the cathode. The electrode through which conventional current flows the other way, into the device, is termed an anode. Charge flow Conventional current flows from cathode to anode outside the cell or device (with electrons moving in the opposite direction), regardless of the cell or device type and operating mode. Cathode polarity ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolytic Cell
An electrolytic cell is an electrochemical cell that utilizes an external source of electrical energy to force a chemical reaction that would otherwise not occur. The external energy source is a voltage applied between the cell's two electrodes; an anode (positively charged electrode) and a cathode (negatively charged electrode), which are immersed in an electrolyte solution. This is in contrast to a galvanic cell, which itself is a source of electrical energy and the foundation of a battery. The net reaction taking place in an electrolytic cell is a non-spontaneous reaction (reverse of a spontaneous reaction), i.e., the Gibbs free energy is +ve, while the net reaction taking place in a galvanic cell is a spontaneous reaction, i.e., the Gibbs free energy is - ve. Principles In an electrolytic cell, a current passes through the cell by an external voltage, causing a non-spontaneous chemical reaction to proceed. In a galvanic cell, the progress of a spontaneous chemical reacti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cathodic
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional current describes the direction in which positive charges move. Electrons, which are the carriers of current in most electrical systems, have a negative electrical charge, so the movement of electrons is ''opposite'' to that of the conventional current flow: this means that electrons flow ''into'' the device's cathode from the external circuit. For example, the end of a household battery marked with a + (plus) is the cathode. The electrode through which conventional current flows the other way, into the device, is termed an anode. Charge flow Conventional current flows from cathode to anode outside the cell or device (with electrons moving in the opposite direction), regardless of the cell or device type and operating mode. Cathode polarity w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electroforming
Electroforming is a metal forming process in which parts are fabricated through electrodeposition on a model, known in the industry as a mandrel. Conductive (metallic) mandrels are treated to create a mechanical parting layer, or are chemically passivated to limit electroform adhesion to the mandrel and thereby allow its subsequent separation. Non-conductive (glass, silicon, plastic) mandrels require the deposition of a conductive layer prior to electrodeposition. Such layers can be deposited chemically, or using vacuum deposition techniques (e.g., gold sputtering). The outer surface of the mandrel forms the inner surface of the form. The process involves passing direct current through an electrolyte containing salts of the metal being electroformed. The anode is the solid metal being electroformed, and the cathode is the mandrel, onto which the electroform gets plated (deposited). The process continues until the required electroform thickness is achieved. The mandrel is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
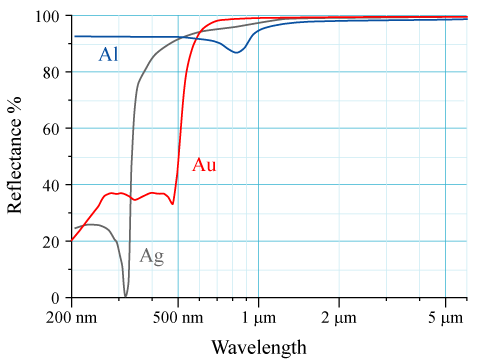
Reflectivity
The reflectance of the surface of a material is its effectiveness in Reflection (physics), reflecting radiant energy. It is the fraction of incident electromagnetic power that is reflected at the boundary. Reflectance is a component of the response of the electronic structure of the material to the electromagnetic field of light, and is in general a function of the frequency, or wavelength, of the light, its polarization, and the angle of incidence (optics), angle of incidence. The dependence of reflectance on the wavelength is called a ''reflectance spectrum'' or ''spectral reflectance curve''. Mathematical definitions Hemispherical reflectance The ''hemispherical reflectance'' of a surface, denoted , is defined as R = \frac, where is the radiant flux ''reflected'' by that surface and is the radiant flux ''received'' by that surface. Spectral hemispherical reflectance The ''spectral hemispherical reflectance in frequency'' and ''spectral hemispherical reflectance in wavelength ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electropolishing
Electropolishing principle: 1. Electrolyte 2. Cathode 3. Workpiece to polish (Anode) 4. Particle moving from the work-piece to the cathode 5. Surface before polishing 6. Surface after polishing Electropolishing, also known as electrochemical polishing, anodic polishing, or electrolytic polishing (especially in the metallography field), is an electrochemical process that removes material from a metallic workpiece, reducing the surface roughness by levelling micro-peaks and valleys, improving the surface finish. Electropolishing is often compared to, but distinctly different from, electrochemical machining. It is used to polish, passivate, and deburr metal parts. It is often described as the reverse of electroplating. It may be used in lieu of abrasive fine polishing in microstructural preparation. Mechanism Typically, the workpiece is immersed in a temperature-controlled bath of electrolyte and serves as the anode; it is connected to the positive terminal of a DC powe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver Chloride Electrode
A silver chloride electrode is a type of reference electrode, commonly used in Electrochemistry, electrochemical measurements. For environmental reasons it has widely replaced the saturated calomel electrode. For example, it is usually the internal reference electrode in pH meters and it is often used as reference in reduction potential measurements. As an example of the latter, the silver chloride electrode is the most commonly used reference electrode for testing cathodic protection corrosion control systems in sea water environments. The electrode functions as a reversible redox electrode and the equilibrium is between the solid (s) silver metal (Ag(s)) and its solid salt—silver chloride (AgCl(s), also called silver(I) chloride) in a chloride solution of a given concentration. In electrochemistry, electrochemical cell notation, the silver chloride electrode is written as, ''e.g.'', for an electrolyte solution of KCl 3 M: : \ , \ \ , \ KCl(aq) \ (3M) The corresponding ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pronunciation of the word "chloride" is . Chloride salts such as sodium chloride are often soluble in water.Green, John, and Sadru Damji. "Chapter 3." ''Chemistry''. Camberwell, Vic.: IBID, 2001. Print. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating liquid flow in and out of cells. Other examples of ionic chlorides include potassium chloride (), calcium chloride (), and ammonium chloride (). Examples of covalent chlorides include methyl chloride (), carbon tetrachloride (), sulfuryl chloride (), and monochloramine (). Electronic properties A chloride ion (diameter 167 pm) is much larger than a chlorine atom (diameter 99 pm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver Chloride
Silver chloride is an inorganic chemical compound with the chemical formula Ag Cl. This white crystalline solid is well known for its low solubility in water and its sensitivity to light. Upon illumination or heating, silver chloride converts to silver (and chlorine), which is signaled by grey to black or purplish coloration in some samples. AgCl occurs naturally as the mineral chlorargyrite. It is produced by a metathesis reaction for use in photography and in pH meters as electrodes. Preparation Silver chloride is unusual in that, unlike most chloride salts, it has very low solubility. It is easily synthesized by metathesis: combining an aqueous solution of silver nitrate (which is soluble) with a soluble chloride salt, such as sodium chloride (which is used industrially as a method of producing AgCl), or cobalt(II) chloride. The silver chloride that forms will precipitate immediately. : : It can also be produced by the reaction of silver metal and aqua regia; howev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anodizing
Anodizing is an electrolytic passivation process used to increase the thickness of the natural oxide layer on the surface of metal parts. The process is called ''anodizing'' because the part to be treated forms the anode electrode of an electrolytic cell. Anodizing increases resistance to corrosion and wear, and provides better adhesion for paint primers and glues than bare metal does. Anodic films can also be used for several cosmetic effects, either with thick porous coatings that can absorb dyes or with thin transparent coatings that add reflected light wave interference effects. Anodizing is also used to prevent galling of threaded components and to make dielectric films for electrolytic capacitors. Anodic films are most commonly applied to protect aluminium alloys, although processes also exist for titanium, zinc, magnesium, niobium, zirconium, hafnium, and tantalum. Iron or carbon steel metal exfoliates when oxidized under neutral or alkaline micro-electrolytic condit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anodic
An anode usually is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, which is usually an electrode of the device through which conventional current leaves the device. A common mnemonic is ACID, for "anode current into device". The direction of conventional current (the flow of positive charges) in a circuit is opposite to the direction of electron flow, so (negatively charged) electrons flow from the anode of a galvanic cell, into an outside or external circuit connected to the cell. For example, the end of a household battery marked with a "+" is the cathode (while discharging). In both a galvanic cell and an electrolytic cell, the anode is the electrode at which the oxidation reaction occurs. In a galvanic cell the anode is the wire or plate having excess negative charge as a result of the oxidation reaction. In an electrolytic cell, the anode is the wire or plate upon which excess positive charge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |