|
Electrophilic Amination
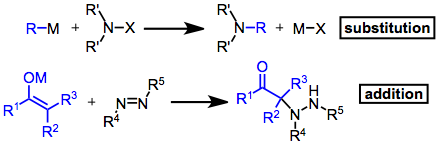
Electrophilic amination is a chemical process involving the formation of a carbon–nitrogen bond through the reaction of a nucleophilic carbanion with an electrophilic source of nitrogen. Introduction Electrophilic amination reactions can be classified as either additions or substitutions. Although the resulting product is not always an amine, these reactions are unified by the formation of a carbon–nitrogen bond and the use of an electrophilic aminating agent. A wide variety of electrophiles have been used; for substitutions, these are most commonly amines substituted with electron-withdrawing groups: chloramines, hydroxylamines, hydrazines, and oxaziridines, for instance. Addition reactions have employed imines, oximes, azides, azo compounds, and others. : Mechanism and stereochemistry Prevailing mechanisms A nitrogen bound to both a good electrofuge and a good nucleofuge is known as a nitrenoid (for its resemblance to a nitrene). Nitrenes lack a full octet of electrons are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloramine
Chloramines refer to derivatives of ammonia and organic amines wherein one or more N-H bonds have been replaced by N-Cl bonds. Two classes of compounds are considered: inorganic chloramines and organic chloramines. Inorganic chloramines Inorganic chloramines comprise three compounds: monochloramine (NH2Cl), dichloramine (NHCl2), and nitrogen trichloride (NCl3). Monochloramine is of broad significance as a disinfectant for water. Organic chloramines 144px, ''N''-Chloropiperidine is a rare example of an organic chloramine. 144px, Chloramine-T is often referred to as a chloramine, but it is really a salt (CH3C6H4SO2NClNa) derived from a chloramine. Organic chloramines feature the NCl functional group attached to an organic substituent. Examples include ''N''-chloromorpholine (ClN(CH2CH2)2O), ''N''-chloropiperidine, and ''N''-chloroquinuclidinium chloride. Chloramines are commonly produced by the action of bleach on secondary amines: :R2NH + NaOCl → R2NCl + NaOH ''Te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enol Ether
In organic chemistry an enol ether is an alkene with an alkoxy substituent. The general structure is R2C=CR-OR where R = H, alkyl or aryl. A common subfamily of enol ethers are vinyl ethers, with the formula ROCH=CH2. Important enol ethers include the reagent 3,4-dihydropyran and the monomers methyl vinyl ether and ethyl vinyl ether. Reactions and uses Akin to enamines, enol ethers are electron-rich alkenes by virtue of the electron-donation from the heteroatom via pi-bonding. Enol ethers have oxonium ion character. By virtue of their bonding situation, enol ethers display distinctive reactivity. In comparison with simple alkenes, enol ethers exhibit enhanced susceptibility to attack by electrophiles such as Bronsted acids. Similarly, they undergo inverse demand Diels-Alder reactions. The reactivity of enol ethers is highly dependent on the presence of substituents alpha to oxygen. The vinyl ethers are susceptible to polymerization to give polyvinyl ethers. Some vinyl ethers al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EA Siloxane
Electronic Arts Inc. (EA) is an American video game company headquartered in Redwood City, California. Founded in May 1982 by Apple employee Trip Hawkins, the company was a pioneer of the early home computer game industry and promoted the designers and programmers responsible for its games as "software artists." EA published numerous games and some productivity software for personal computers, all of which were developed by external individuals or groups until 1987's ''Skate or Die!''. The company shifted toward internal game studios, often through acquisitions, such as Distinctive Software becoming EA Canada in 1991. Currently, EA develops and publishes games of established franchises, including ''Battlefield'', ''Need for Speed'', ''The Sims'', ''Medal of Honor'', ''Command & Conquer'', ''Dead Space'', ''Mass Effect'', ''Dragon Age'', ''Army of Two'', ''Apex Legends'', and ''Star Wars'', as well as the EA Sports titles ''FIFA'', ''Madden NFL'', ''NBA Live'', ''NHL'', and ' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinyllithium
Vinyllithium is an organolithium compound with the formula LiC2H3. A colorless or white solid, it is encountered mainly as a solution in tetrahydrofuran (THF). It is a reagent in synthesis of organic compounds.. Preparation and structure Solutions of vinyllithium are prepared by lithium-halogen exchange reactions. A halide-free route entails reaction of tetravinyltin with butyllithium Butyllithium may refer to one of 5 isomeric organolithium reagents of which 3 are commonly used in chemical synthesis: * ''n''-Butyllithium, abbreviated BuLi or nBuLi * ''sec''-Butyllithium, abbreviated ''sec''-BuLi or sBuLi, has 2 stereoisomers, ...: :Sn(CH=CH2)4 + 4 BuLi → SnBu4 + 4 LiCH=CH2 The reaction of ethylene and lithium affords vinyl lithium and lithium hydride, together with other organolithium compounds, Like most organolithium compounds, vinyllithium crystallizes from THF as a cluster compound as a cubane-type cluster. Reactions Vinyllithium is used to install vi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imines
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions. Structure For ketimines and aldimines, respectively, the five core atoms (C2C=NX and C(H)C=NX, X = H or C) are coplanar. Planarity results from the sp2-hybridization of the mutually double-bonded carbon and the nitrogen atoms. The C=N distance is 1.29-1.31 Å for nonconjugated imines and 1.35 Å for conjugated imines. By contrast, C-N distances in amines and nitriles are 1.47 and 1.16 Å, respectively. Rotation about the C=N bond is slow. Using NMR spectroscopy, both E- and Z-isomers of aldimines have been detected. Owing to steric effects, the E isomer is favored. Nomenclature and classification The term "imine" was coined ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diazo
The diazo group is an organic moiety consisting of two linked nitrogen atoms ( azo) at the terminal position. Overall charge neutral organic compounds containing the diazo group bound to a carbon atom are called diazo compounds or diazoalkanes and are described by the general structural formula R2C=N+=N–. The simplest example of a diazo compound is diazomethane, CH2N2. Diazo compounds (R2C=N2) should not be confused with azo compounds of the type R-N=N-R or with diazonium compounds of the type R-N2+. Structure The electronic structure of diazo compounds is characterized by π electron density delocalized over the α-carbon and two nitrogen atoms, along with an orthogonal π system with electron density delocalized over only the terminal nitrogen atoms. Because all octet rule-satisfying resonance forms of diazo compounds have formal charges, they are members of a class of compounds known as 1,3-dipoles. Some of the most stable diazo compounds are α-diazo-β-diketones an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)
