|
Eigenstates
In quantum physics, a quantum state is a mathematical entity that provides a probability distribution for the outcomes of each possible measurement on a system. Knowledge of the quantum state together with the rules for the system's evolution in time exhausts all that can be predicted about the system's behavior. A mixture of quantum states is again a quantum state. Quantum states that cannot be written as a mixture of other states are called pure quantum states, while all other states are called mixed quantum states. A pure quantum state can be represented by a ray in a Hilbert space over the complex numbers, while mixed states are represented by density matrices, which are positive semidefinite operators that act on Hilbert spaces. Pure states are also known as state vectors or wave functions, the latter term applying particularly when they are represented as functions of position or momentum. For example, when dealing with the energy spectrum of the electron in a hydrog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
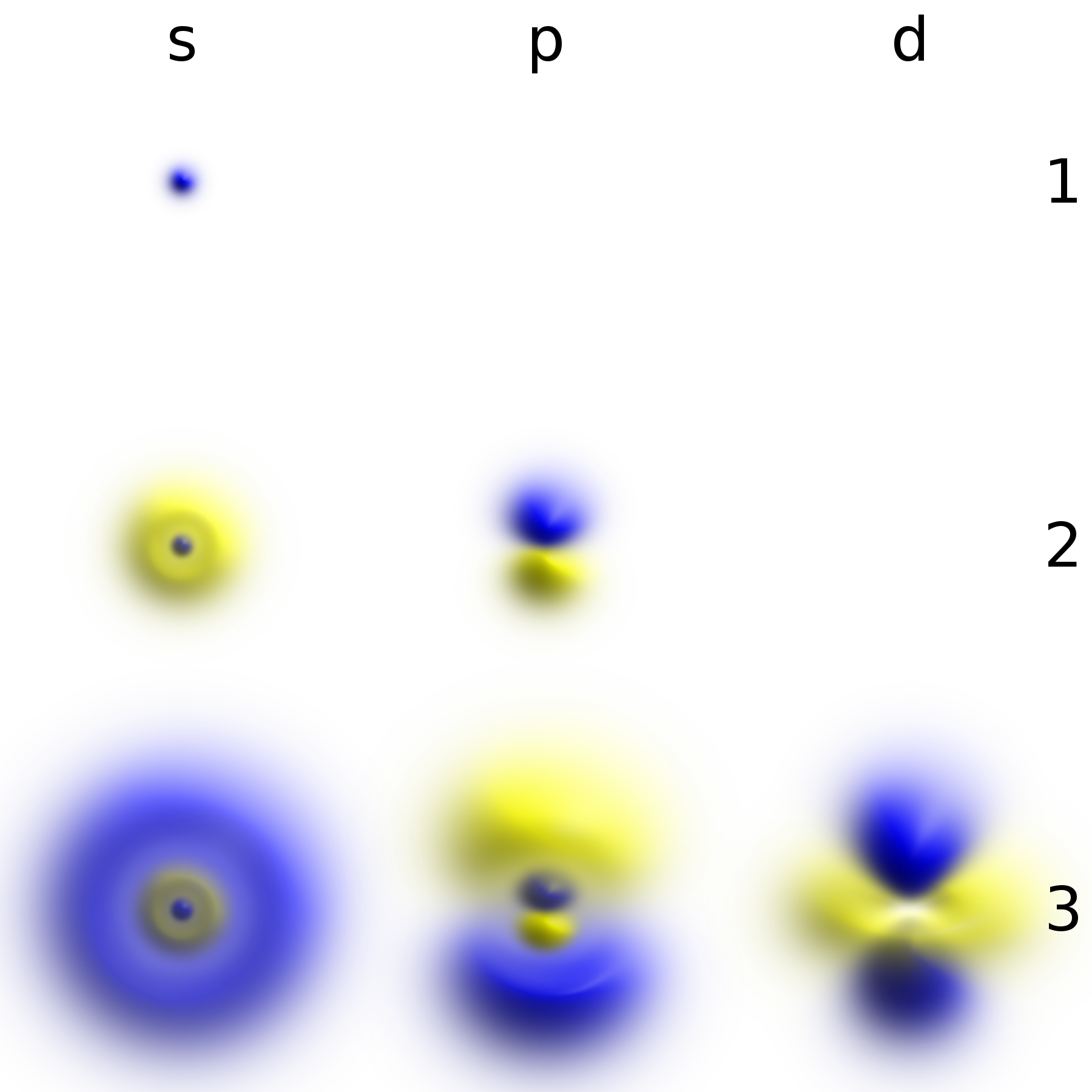
Hydrogen Atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the baryonic mass of the universe. In everyday life on Earth, isolated hydrogen atoms (called "atomic hydrogen") are extremely rare. Instead, a hydrogen atom tends to combine with other atoms in compounds, or with another hydrogen atom to form ordinary ( diatomic) hydrogen gas, H2. "Atomic hydrogen" and "hydrogen atom" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms). Atomic spectroscopy shows that there is a discrete infinite set of states in which a hydrogen (or any) atom can exist, contrary to the predictions of classical physics. Attempts to develop a the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Physics
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, quantum field theory, quantum technology, and quantum information science. Classical physics, the collection of theories that existed before the advent of quantum mechanics, describes many aspects of nature at an ordinary ( macroscopic) scale, but is not sufficient for describing them at small (atomic and subatomic) scales. Most theories in classical physics can be derived from quantum mechanics as an approximation valid at large (macroscopic) scale. Quantum mechanics differs from classical physics in that energy, momentum, angular momentum, and other quantities of a bound system are restricted to discrete values ( quantization); objects have characteristics of both particles and waves (wave–particle duality); and there are limits to ho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wave Function
A wave function in quantum physics is a mathematical description of the quantum state of an isolated quantum system. The wave function is a complex-valued probability amplitude, and the probabilities for the possible results of measurements made on the system can be derived from it. The most common symbols for a wave function are the Greek letters and (lower-case and capital psi, respectively). The wave function is a function of the degrees of freedom corresponding to some maximal set of commuting observables. Once such a representation is chosen, the wave function can be derived from the quantum state. For a given system, the choice of which commuting degrees of freedom to use is not unique, and correspondingly the domain of the wave function is also not unique. For instance, it may be taken to be a function of all the position coordinates of the particles over position space, or the momenta of all the particles over momentum space; the two are related by a Fourier ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spin (physics)
Spin is a conserved quantity carried by elementary particles, and thus by composite particles (hadrons) and atomic nuclei. Spin is one of two types of angular momentum in quantum mechanics, the other being ''orbital angular momentum''. The orbital angular momentum operator is the quantum-mechanical counterpart to the classical angular momentum of orbital revolution and appears when there is periodic structure to its wavefunction as the angle varies. For photons, spin is the quantum-mechanical counterpart of the polarization of light; for electrons, the spin has no classical counterpart. The existence of electron spin angular momentum is inferred from experiments, such as the Stern–Gerlach experiment, in which silver atoms were observed to possess two possible discrete angular momenta despite having no orbital angular momentum. The existence of the electron spin can also be inferred theoretically from the spin–statistics theorem and from the Pauli exclusion principle—and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Singlet State
In quantum mechanics, a singlet state usually refers to a system in which all electrons are paired. The term 'singlet' originally meant a linked set of particles whose net angular momentum is zero, that is, whose overall spin quantum number s=0. As a result, there is only one spectral line of a singlet state. In contrast, a doublet state contains one unpaired electron and shows splitting of spectral lines into a doublet; and a triplet state has two unpaired electrons and shows threefold splitting of spectral lines. History Singlets and the related spin concepts of doublets and triplets occur frequently in atomic physics and nuclear physics, where one often needs to determine the total spin of a collection of particles. Since the only observed fundamental particle with zero spin is the extremely inaccessible Higgs boson, singlets in everyday physics are necessarily composed of sets of particles whose individual spins are non-zero, e.g. or 1. The origin of the term "singlet" ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Principal Quantum Number
In quantum mechanics, the principal quantum number (symbolized ''n'') is one of four quantum numbers assigned to each electron in an atom to describe that electron's state. Its values are natural numbers (from 1) making it a discrete variable. Apart from the principal quantum number, the other quantum numbers for bound electrons are the azimuthal quantum number ''ℓ'', the magnetic quantum number ''ml'', and the spin quantum number ''s''. Overview and history As ''n'' increases, the electron is also at a higher energy and is, therefore, less tightly bound to the nucleus. For higher ''n'' the electron is farther from the nucleus, on average. For each value of ''n'' there are ''n'' accepted ''ℓ'' (azimuthal) values ranging from 0 to ''n'' − 1 inclusively, hence higher-''n'' electron states are more numerous. Accounting for two states of spin, each ''n''- shell can accommodate up to 2''n''2 electrons. In a simplistic one-electron model described bel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnetic Quantum Number
In atomic physics, the magnetic quantum number () is one of the four quantum numbers (the other three being the principal, azimuthal, and spin) which describe the unique quantum state of an electron. The magnetic quantum number distinguishes the orbitals available within a subshell, and is used to calculate the azimuthal component of the orientation of orbital in space. Electrons in a particular subshell (such as s, p, d, or f) are defined by values of (0, 1, 2, or 3). The value of can range from to , including zero. Thus the s, p, d, and f subshells contain 1, 3, 5, and 7 orbitals each, with values of within the ranges 0, ±1, ±2, ±3 respectively. Each of these orbitals can accommodate up to two electrons (with opposite spins), forming the basis of the periodic table. Derivation There is a set of quantum numbers associated with the energy states of the atom. The four quantum numbers n, \ell, m_\ell, and s specify the complete quantum state of a single electron in an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Measurement In Quantum Mechanics
In quantum physics, a measurement is the testing or manipulation of a physical system to yield a numerical result. The predictions that quantum physics makes are in general probabilistic. The mathematical tools for making predictions about what measurement outcomes may occur were developed during the 20th century and make use of linear algebra and functional analysis. Quantum physics has proven to be an empirical success and to have wide-ranging applicability. However, on a more philosophical level, debates continue about the meaning of the measurement concept. Mathematical formalism "Observables" as self-adjoint operators In quantum mechanics, each physical system is associated with a Hilbert space, each element of which represents a possible state of the physical system. The approach codified by John von Neumann represents a measurement upon a physical system by a self-adjoint operator on that Hilbert space termed an "observable". These observables play the role of measurable ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Superposition
Quantum superposition is a fundamental principle of quantum mechanics. It states that, much like waves in classical physics, any two (or more) quantum states can be added together ("superposed") and the result will be another valid quantum state; and conversely, that every quantum state can be represented as a sum of two or more other distinct states. Mathematically, it refers to a property of solutions to the Schrödinger equation; since the Schrödinger equation is linear, any linear combination of solutions will also be a solution(s) . An example of a physically observable manifestation of the wave nature of quantum systems is the interference peaks from an electron beam in a double-slit experiment. The pattern is very similar to the one obtained by diffraction of classical waves. Another example is a quantum logical qubit state, as used in quantum information processing, which is a quantum superposition of the "basis states" , 0 \rangle and , 1 \rangle . Here ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Entanglement
Quantum entanglement is the phenomenon that occurs when a group of particles are generated, interact, or share spatial proximity in a way such that the quantum state of each particle of the group cannot be described independently of the state of the others, including when the particles are separated by a large distance. The topic of quantum entanglement is at the heart of the disparity between classical and quantum physics: entanglement is a primary feature of quantum mechanics not present in classical mechanics. Measurements of physical properties such as position, momentum, spin, and polarization performed on entangled particles can, in some cases, be found to be perfectly correlated. For example, if a pair of entangled particles is generated such that their total spin is known to be zero, and one particle is found to have clockwise spin on a first axis, then the spin of the other particle, measured on the same axis, is found to be anticlockwise. However, this behavior gives ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schrödinger–HJW Theorem
In quantum information theory and quantum optics, the Schrödinger–HJW theorem is a result about the realization of a mixed state of a quantum system as an ensemble of pure quantum states and the relation between the corresponding purifications of the density operators. The theorem is named after physicists and mathematicians Erwin Schrödinger, Lane P. Hughston, Richard Jozsa and William Wootters William "Bill" Kent Wootters () is an American theoretical physicist, and one of the founders of the field of quantum information theory. In a 1982 joint paper with Wojciech H. Zurek, Wootters proved the no cloning theorem, at the same time as D .... The result was also found independently (albeit partially) by Nicolas Gisin, and by Nicolas Hadjisavvas building upon work by Ed Jaynes, while a significant part of it was likewise independently discovered by N. David Mermin. Thanks to its complicated history, it is also known by various other names such as the GHJW theorem, the HJ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trace (linear Algebra)
In linear algebra, the trace of a square matrix , denoted , is defined to be the sum of elements on the main diagonal (from the upper left to the lower right) of . The trace is only defined for a square matrix (). It can be proved that the trace of a matrix is the sum of its (complex) eigenvalues (counted with multiplicities). It can also be proved that for any two matrices and . This implies that similar matrices have the same trace. As a consequence one can define the trace of a linear operator mapping a finite-dimensional vector space into itself, since all matrices describing such an operator with respect to a basis are similar. The trace is related to the derivative of the determinant (see Jacobi's formula). Definition The trace of an square matrix is defined as \operatorname(\mathbf) = \sum_^n a_ = a_ + a_ + \dots + a_ where denotes the entry on the th row and th column of . The entries of can be real numbers or (more generally) complex numbers. The trace is not ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |