|
Dynamic Mechanical Analysis
Dynamic mechanical analysis (abbreviated DMA) is a technique used to study and characterize materials. It is most useful for studying the viscoelastic behavior of polymers. A sinusoidal stress is applied and the strain in the material is measured, allowing one to determine the complex modulus. The temperature of the sample or the frequency of the stress are often varied, leading to variations in the complex modulus; this approach can be used to locate the glass transition temperature of the material, as well as to identify transitions corresponding to other molecular motions. Theory Viscoelastic properties of materials Polymers composed of long molecular chains have unique viscoelastic properties, which combine the characteristics of elastic solids and Newtonian fluids. The classical theory of elasticity describes the mechanical properties of elastic solid where stress is proportional to strain in small deformations. Such response of stress is independent of strain rate. The c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Analysis
Thermal analysis is a branch of materials science where the properties of materials are studied as they change with temperature. Several methods are commonly used – these are distinguished from one another by the property which is measured: * Dielectric thermal analysis: dielectric permittivity and loss factor * Differential thermal analysis: temperature difference versus temperature or time * Differential scanning calorimetry: heat flow changes versus temperature or time * Dilatometry: volume changes with temperature change * Dynamic mechanical analysis: measures storage modulus (stiffness) and loss modulus (damping) versus temperature, time and frequency * Evolved gas analysis: analysis of gases evolved during heating of a material, usually decomposition products * Isothermal titration calorimetry * Isothermal microcalorimetry * Laser flash analysis: thermal diffusivity and thermal conductivity * Thermogravimetric analysis: mass change versus temperature or time * Ther ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds. Thailand, Malaysia, and Indonesia are three of the leading rubber producers. Types of polyisoprene that are used as natural rubbers are classified as elastomers. Currently, rubber is harvested mainly in the form of the latex from the rubber tree (''Hevea brasiliensis'') or others. The latex is a sticky, milky and white colloid drawn off by making incisions in the bark and collecting the fluid in vessels in a process called "tapping". The latex then is refined into the rubber that is ready for commercial processing. In major areas, latex is allowed to coagulate in the collection cup. The coagulated lumps are collected and processed into dry forms for sale. Natural rubber is used extensively in many applications and products, either alone or in combination ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polycarbonate
Polycarbonates (PC) are a group of thermoplastic polymers containing carbonate groups in their chemical structures. Polycarbonates used in engineering are strong, tough materials, and some grades are optically transparent. They are easily worked, molded, and thermoformed. Because of these properties, polycarbonates find many applications. Polycarbonates do not have a unique resin identification code (RIC) and are identified as "Other", 7 on the RIC list. Products made from polycarbonate can contain the precursor monomer bisphenol A (BPA). Structure Carbonate esters have planar OC(OC)2 cores, which confers rigidity. The unique O=C bond is short (1.173 Å in the depicted example), while the C-O bonds are more ether-like (the bond distances of 1.326 Å for the example depicted). Polycarbonates received their name because they are polymers containing carbonate groups (−O−(C=O)−O−). A balance of useful features, including temperature resistance, impact resistance and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Semi-crystalline Polymer
Crystallization of polymers is a process associated with partial alignment of their molecular chains. These chains fold together and form ordered regions called lamellae, which compose larger spheroidal structures named spherulites. Polymers can crystallize upon cooling from melting, mechanical stretching or solvent evaporation. Crystallization affects optical, mechanical, thermal and chemical properties of the polymer. The degree of crystallinity is estimated by different analytical methods and it typically ranges between 10 and 80%, with crystallized polymers often called "semi-crystalline". The properties of semi-crystalline polymers are determined not only by the degree of crystallinity, but also by the size and orientation of the molecular chains. Crystallization mechanisms Solidification from the melt Polymers are composed of long molecular chains which form irregular, entangled coils in the melt. Some polymers retain such a disordered structure upon freezing and readily ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermomechanical Analysis
Thermomechanical analysis (TMA) is a technique used in thermal analysis, a branch of materials science which studies the properties of materials as they change with temperature. Thermomechanical analysis is a subdiscipline of the thermomechanometry (TM) technique. Related techniques and terminology Thermomechanometry is the measurement of a change of a dimension or a mechanical property of the sample while it is subjected to a temperature regime. An associated thermoanalytical method is thermomechanical analysis. A special related technique is thermodilatometry (TD), the measurement of a change of a dimension of the sample with a negligible force acting on the sample while it is subjected to a temperature regime. The associated thermoanalytical method is thermodilatometric analysis (TDA). TDA is often referred to as zero force TMA. The temperature regime may be heating, cooling at a rate of temperature change that can include stepwise temperature changes, linear rate of change ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inertia
Inertia is the idea that an object will continue its current motion until some force causes its speed or direction to change. The term is properly understood as shorthand for "the principle of inertia" as described by Newton in his first law of motion. After some other definitions, Newton states in his first law of motion: The word "perseveres" is a direct translation from Newton's Latin. Other, less forceful terms such as "to continue" or "to remain" are commonly found in modern textbooks. The modern use follows from some changes in Newton's original mechanics (as stated in the ''Principia'') made by Euler, d'Alembert, and other Cartesians. The term inertia comes from the Latin word ''iners'', meaning idle, sluggish. The term inertia may also refer to the resistance of any physical object to a change in its velocity. This includes changes to the object's speed or direction of motion. An aspect of this property is the tendency of objects to keep moving in a straight lin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Linear Variable Differential Transformer
The linear variable differential transformer (LVDT) (also called linear variable displacement transformer, linear variable displacement transducer, or simply differential transformer) is a type of electrical transformer used for measuring linear displacement (position). A counterpart to this device that is used for measuring rotary displacement is called a rotary variable differential transformer ( RVDT). Introduction LVDTs are robust, absolute linear position/displacement transducers; inherently frictionless, they have a virtually infinite cycle life when properly used. As AC operated LVDTs do not contain any electronics, they can be designed to operate at cryogenic temperatures or up to 1200 °F (650 °C), in harsh environments, and under high vibration and shock levels. LVDTs have been widely used in applications such as power turbines, hydraulics, automation, aircraft, satellites, nuclear reactors, and many others. These transducers have low hysteresis and excel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schematic Of DMA
A schematic, or schematic diagram, is a designed representation of the elements of a system using abstract, graphic symbols rather than realistic pictures. A schematic usually omits all details that are not relevant to the key information the schematic is intended to convey, and may include oversimplified elements in order to make this essential meaning easier to grasp, as well as additional organization of the information. For example, a subway map intended for passengers may represent a subway station with a dot. The dot is not intended to resemble the actual station at all but aims to give the viewer information without unnecessary visual clutter. A schematic diagram of a chemical process uses symbols in place of detailed representations of the vessels, piping, valves, pumps, and other equipment that compose the system, thus emphasizing the functions of the individual elements and the interconnections among them and suppresses their physical details. In an electronic circuit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steric
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions and molecules. Steric effects complement electronic effects, which dictate the shape and reactivity of molecules. Steric repulsive forces between overlapping electron clouds result in structured groupings of molecules stabilized by the way that opposites attract and like charges repel. Steric hindrance Steric hindrance is a consequence of steric effects. Steric hindrance is the slowing of chemical reactions due to steric bulk. It is usually manifested in ''intermolecular reactions'', whereas discussion of steric effects often focus on ''intramolecular interactions''. Steric hindrance is often exploited to control selectivity, such as slowing unwanted side-reactions. Steric hindrance between adjacent groups can also affect torsional ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
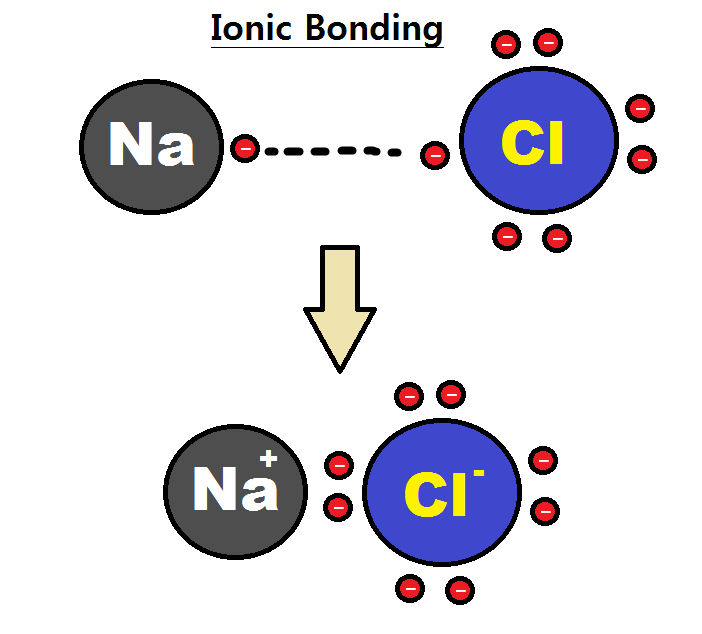
Intramolecular Force
An intramolecular force (or primary forces) is any force that binds together the atoms making up a molecule or compound, not to be confused with intermolecular forces, which are the forces present between molecules. The subtle difference in the name comes from the Latin roots of English with inter meaning ''between or among'' and intra meaning ''inside''. Chemical bonds are considered to be intramolecular forces which are often stronger than intermolecular forces present between non-bonding atoms or molecules. Types The classical model identifies three main types of chemical bonds — ionic, covalent, and metallic — distinguished by the degree of charge separation between participating atoms. The characteristics of the bond formed can be predicted by the properties of constituent atoms, namely electronegativity. They differ in the magnitude of their bond enthalpies, a measure of bond strength, and thus affect the physical and chemical properties of compounds in different ways. % ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intermolecular
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction or repulsion which act between atoms and other types of neighbouring particles, e.g. atoms or ions. Intermolecular forces are weak relative to intramolecular forces – the forces which hold a molecule together. For example, the covalent bond, involving sharing electron pairs between atoms, is much stronger than the forces present between neighboring molecules. Both sets of forces are essential parts of force fields frequently used in molecular mechanics. The investigation of intermolecular forces starts from macroscopic observations which indicate the existence and action of forces at a molecular level. These observations include non-ideal-gas thermodynamic behavior reflected by virial coefficients, vapor pressure, viscosity, superficial tension, and absorption data. The first reference to the nature of microscopic for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |