|
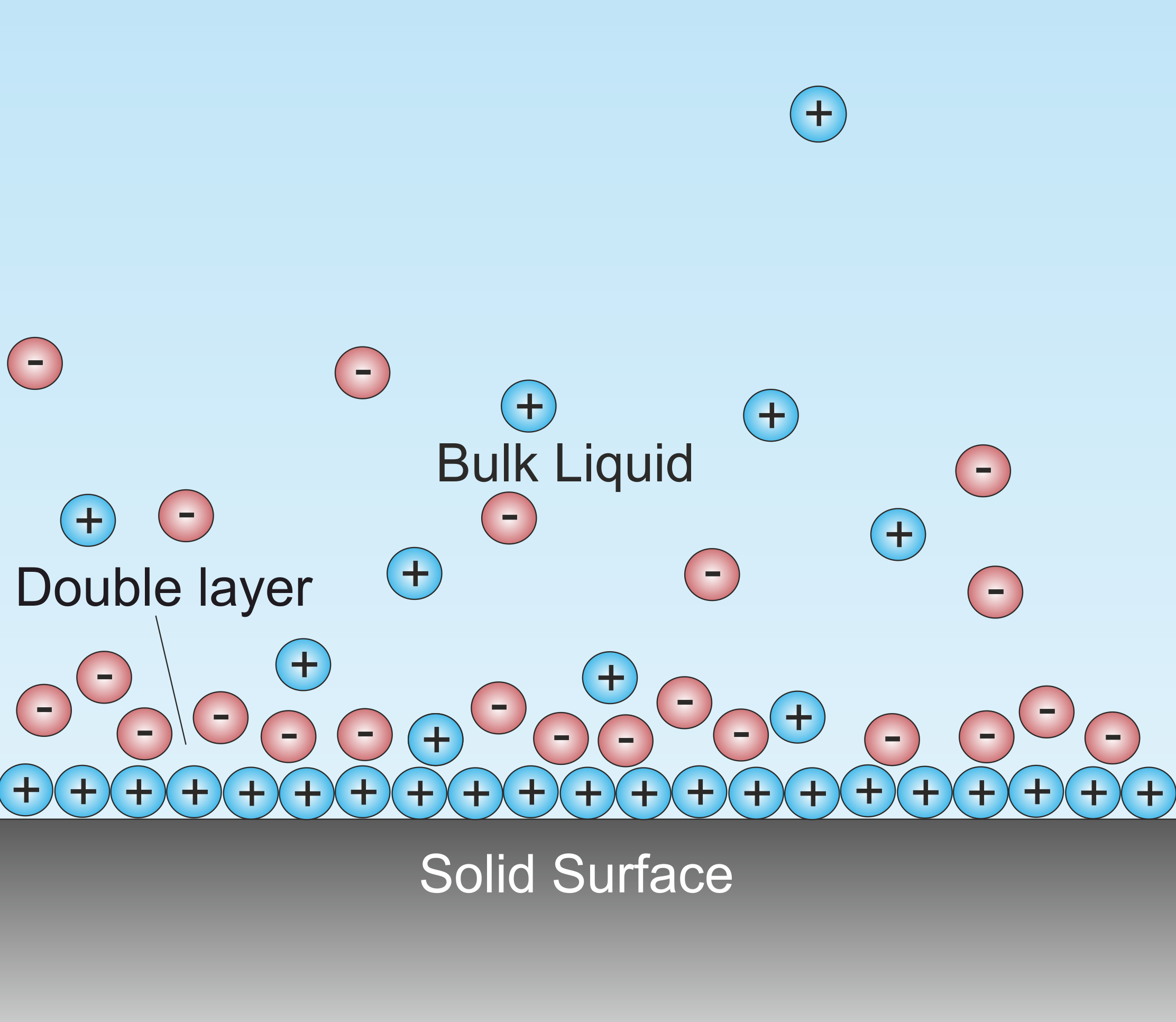
Double Layer (electrode)
A double layer (DL, also called an electrical double layer, EDL) is a structure that appears on the surface of an object when it is exposed to a fluid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The DL refers to two parallel layers of charge surrounding the object. The first layer, the surface charge (either positive or negative), consists of ions adsorbed onto the object due to chemical interactions. The second layer is composed of ions attracted to the surface charge via the Coulomb force, electrically screening the first layer. This second layer is loosely associated with the object. It is made of free ions that move in the fluid under the influence of electric attraction and thermal motion rather than being firmly anchored. It is thus called the "diffuse layer". Interfacial DLs are most apparent in systems with a large surface area to volume ratio, such as a colloid or porous bodies with particles or pores (respectively) on t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electroacoustic Phenomena
Electroacoustic phenomena arise when ultrasound propagates through a fluid containing ions. The associated particle motion generates electric signals because ions have electric charge. This coupling between ultrasound and electric field is called electroacoustic phenomena. The fluid might be a simple Newtonian liquid, or complex heterogeneous dispersion, emulsion or even a porous body. There are several different electroacoustic effects depending on the nature of the fluid.Dukhin, A.S. and Goetz, P.J. ''Characterization of liquids, nano- and micro- particulates and porous bodies using Ultrasound'', Elsevier, 2017 *Ion vibration current (IVI) and potential, an electric signal that arises when an acoustic wave propagates through a homogeneous fluid. * Streaming vibration current (SVI) and potential, an electric signal that arises when an acoustic wave propagates through a porous body in which the pores are filled with fluid. *Colloid vibration current (CVI) and potential, an electr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
David Chapman (scientist)
David Leonard Chapman FRS (6 December 1869 – 17 January 1958) was an English physical chemist, whose name is associated with the Chapman-Jouguet treatment (on the theory of detonation in gases) and the Gouy-Chapman layer (the surface layer of ions distributed on a charged surface). He was a fellow of Jesus College, Oxford for 37 years, and was in charge there of the last college laboratory at the University of Oxford. Education and early life Chapman was born in Wells, Norfolk but moved with his family to Manchester and attended Manchester Grammar School. He then went to Christ Church, Oxford, obtaining degrees in chemistry (1893, 1st class) and physics (1894, 2nd class). Personal life Campman was by reputation something of a scientific recluse, difficult to dislodge from his laboratory, although he did play a full part in University and College affairs. Away from his teaching and research, he was reserved and somewhat eccentric, but enjoyed golf, cycling and walking. He m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Louis Georges Gouy
Louis Georges Gouy (February 19, 1854 – January 27, 1926) was a French physicist. He is the namesake of the Gouy balance, the Gouy–Chapman electric double layer model (which is a relatively successful albeit limited model that describes the electrical double-layer which finds applications in vast areas of studies from physical chemistry to biophysics) and the Gouy phase. Gouy was born at Vals-les-Bains, Ardèche in 1854. He became a correspondent of the Académie des sciences in 1901, and a member in 1913. Topics investigated His principal scientific work was related to the following subjects: * The propagation velocity of light waves in dispersive media. * Propagation of spherical waves of small radius. * Distant diffraction (angles of dispersion reaching 150°) * Electrostatics: Inductive capacity of dielectrics * Surface charge * Effect of the magnetic field on the discharge in rarefied gases * Electrocapillarity * Emission capacity of absorbent of the coloured flame ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Dipole Moment
The electric dipole moment is a measure of the separation of positive and negative electrical charges within a system, that is, a measure of the system's overall polarity. The SI unit for electric dipole moment is the coulomb-meter (C⋅m). The debye (D) is another unit of measurement used in atomic physics and chemistry. Theoretically, an electric dipole is defined by the first-order term of the multipole expansion; it consists of two equal and opposite charges that are infinitesimally close together, although real dipoles have separated charge.Many theorists predict elementary particles can have very tiny electric dipole moments, possibly without separated charge. Such large dipoles make no difference to everyday physics, and have not yet been observed. (See electron electric dipole moment). However, when making measurements at a distance much larger than the charge separation, the dipole gives a good approximation of the actual electric field. The dipole is represented by a v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a fluid (the ''absorbate'') is dissolved by or permeates a liquid or solid (the ''absorbent''). Adsorption is a '' surface phenomenon'', while absorption involves the whole volume of the material, although adsorption does often precede absorption. The term ''sorption'' encompasses both processes, while ''desorption'' is the reverse of it. Like surface tension, adsorption is a consequence of surface energy. In a bulk material, all the bonding requirements (be they ionic, covalent or metallic) of the constituent atoms of the material are fulfilled by other atoms in the material. However, atoms on the surface of the adsorbent are not wholly surrounded by other adsorbent atoms and therefore can attract adsorbates. The exact nature of the bon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell. The quantity of solute that can dissolve in a specific volume of solvent varies with temperature. Major uses of solvents are in paints, paint removers, inks, and dry cleaning. Specific uses for organic solvents are in dry cleaning (e.g. tetrachloroethylene); as paint thinners (toluene, turpentine); as nail polish removers and solvents of glue (acetone, methyl acetate, ethyl acetate); in spot removers (hexane, petrol ether); in detergents ( citrus terpenes); and in perfumes (ethanol). Solvents find various applications in chemical, pharmaceutical, oil, and gas industries, including in chemical syn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dielectric Constant
The relative permittivity (in older texts, dielectric constant) is the permittivity of a material expressed as a ratio with the electric permittivity of a vacuum. A dielectric is an insulating material, and the dielectric constant of an insulator measures the ability of the insulator to store electric energy in an electrical field. Permittivity is a material's property that affects the Coulomb force between two point charges in the material. Relative permittivity is the factor by which the electric field between the charges is decreased relative to vacuum. Likewise, relative permittivity is the ratio of the capacitance of a capacitor using that material as a dielectric, compared with a similar capacitor that has vacuum as its dielectric. Relative permittivity is also commonly known as the dielectric constant, a term still used but deprecated by standards organizations in engineering as well as in chemistry. Definition Relative permittivity is typically denoted as (sometimes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Differential Capacitance
Differential capacitance in physics, electronics, and electrochemistry is a measure of the voltage-dependent capacitance of a nonlinear capacitor, such as an electrical double layer or a semiconductor diode. It is defined as the derivative of charge with respect to potential. Description In electrochemistry differential capacitance is a parameter introduced for characterizing electrical double layers: : C = \frac where σ is surface charge and ψ is electric surface potential. Capacitance is usually defined as the stored charge between two conducting surfaces separated by a dielectric divided by the voltage between the surfaces. Another definition is the rate of change of the stored charge or surface charge (σ) divided by the rate of change of the voltage between the surfaces or the electric surface potential (ψ). The latter is called the "differential capacitance," but usually the stored charge is directly proportional to the voltage, making the capacitances given by the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are typically not consid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrical Polarity
An electric current is a stream of charged particles, such as electrons or ions, moving through an electrical conductor or space. It is measured as the net rate of flow of electric charge through a surface or into a control volume. The moving particles are called charge carriers, which may be one of several types of particles, depending on the conductor. In electric circuits the charge carriers are often electrons moving through a wire. In semiconductors they can be electrons or Electron hole, holes. In an Electrolyte#Electrochemistry, electrolyte the charge carriers are ions, while in Plasma (physics), plasma, an ionized gas, they are ions and electrons. The International System of Units, SI unit of electric current is the ampere, or ''amp'', which is the flow of electric charge across a surface at the rate of one coulomb per second. The ampere (symbol: A) is an SI base unit. Electric current is measured using a device called an ammeter. Electric currents create magnetic fields ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Charge
Electric charge is the physical property of matter that causes charged matter to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative'' (commonly carried by protons and electrons respectively). Like charges repel each other and unlike charges attract each other. An object with an absence of net charge is referred to as neutral. Early knowledge of how charged substances interact is now called classical electrodynamics, and is still accurate for problems that do not require consideration of quantum effects. Electric charge is a conserved property; the net charge of an isolated system, the amount of positive charge minus the amount of negative charge, cannot change. Electric charge is carried by subatomic particles. In ordinary matter, negative charge is carried by electrons, and positive charge is carried by the protons in the nuclei of atoms. If there are more electrons than protons in a piece of matter, it will have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |