|
Diterpenoids
Diterpenes are a class of chemical compounds composed of four isoprene units, often with the molecular formula C20H32. They are biosynthesized by plants, animals and fungi via the HMG-CoA reductase pathway, with geranylgeranyl pyrophosphate being a primary intermediate. Diterpenes form the basis for biologically important compounds such as retinol, retinal, and phytol. They are known to be antimicrobial and antiinflammatory. Structures As with most terpenes a huge number of potential structures exists, which may be broadly divided according to the number of rings present. Biosynthesis Diterpenes are derived from the addition of one IPP unit to FPP to form geranylgeranyl-pyrophosphate (GGPP). From GGPP, structural diversity is achieved mainly by two classes of enzymes; the diterpene synthases and cytochromes P450. Several diterpenes are produced by plants and cyanobacteria. GGPP is also the precursor for the synthesis of the phytane by the action of the enzyme geranylg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geranylgeranyl Pyrophosphate
Geranylgeranyl pyrophosphate is an intermediate in the biosynthesis of diterpenes and diterpenoids. It is also the precursor to carotenoids, gibberellins, tocopherols, and chlorophylls. It is also a precursor to geranylgeranylated proteins, which is its primary use in human cells. It is formed from farnesyl pyrophosphate by the addition of an isoprene unit from isopentenyl pyrophosphate. In ''Drosophila'', geranylgeranyl pyrophosphate is synthesised by HMG-CoA encoded by the Columbus gene. Geranylgeranyl pyrophosphate is utilised as a chemoattractant for migrating germ cells that have traversed the midgut epithelia. The attractant signal is produced at the gonadal precursors, directing the germ cells to these sites, where they will differentiate into eggs and spermatozoa (sperm). Related compounds * Farnesyl pyrophosphate * Geranylgeraniol * Geranyl pyrophosphate Geranyl pyrophosphate (GPP), also known as geranyl diphosphate (GDP), is the pyrophosphate ester of the terpenoi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stemodene
Stemodene is a labdane-related diterpene whose corresponding terpene synthase has been discovered in rice and subsequently cloned and functionally characterized. The gene responsible for stemodene production has not been found in the completed rice genome, thus suggesting that perhaps other genes are as yet undiscovered in the "completed" genome. Stemarene synthase demonstrates high sequence homology with stemodene synthase, thus accounting for the latter's discovery by Dana Morrone in 2005. Additionally, the corresponding olefin produced by each cyclase A cyclase is an enzyme, almost always a lyase, that catalyzes a chemical reaction to form a cyclic compound. Important cyclase enzymes include: * Adenylyl cyclase, which forms cyclic AMP from adenosine triphosphate (EC 4.6.1.1) ** ADCY1 ** ADCY2 ... shows structural similarities and is derived from the common precursor of ''syn''-copalyl diphosphate. References Diterpenes Cyclopentanes {{Organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phytol
Phytol (florasol, phytosol) is an acyclic hydrogenated diterpene alcohol that can be used as a precursor for the manufacture of synthetic forms of vitamin E and vitamin K1. In ruminants, the gut fermentation of ingested plant materials liberates phytol, a constituent of chlorophyll, which is then converted to phytanic acid and stored in fats. In shark liver it yields pristane. Human pathology Refsum disease (also known as adult Refsum disease) is an autosomal recessive disorder that results in the accumulation of toxic stores of phytanic acid in tissues and frequently manifests as a variable combination of peripheral polyneuropathy, cerebellar ataxia, retinitis pigmentosa, anosmia, and hearing loss. Although humans cannot derive phytanic acid from chlorophyll, they can convert free phytol into phytanic acid. Thus, patients with Refsum disease should limit their intake of phytanic acid and free phytol. The amount of free phytol in numerous food products has been reported. Roles ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Retinol
Retinol, also called vitamin A1, is a fat-soluble vitamin in the vitamin A family found in food and used as a dietary supplement. As a supplement it is used to treat and prevent vitamin A deficiency, especially that which results in xerophthalmia. In regions where deficiency is common, a single large dose is recommended to those at high risk twice a year. It is also used to reduce the risk of complications in measles patients. It is taken by mouth or by injection into a muscle. Retinol at normal doses is well tolerated. High doses may cause enlargement of the liver, dry skin, and hypervitaminosis A. High doses during pregnancy may harm the fetus. The body converts retinol to retinal and retinoic acid, through which it acts. Dietary sources include fish, dairy products, and meat. Retinol was discovered in 1909, isolated in 1931, and first made in 1947. It is on the World Health Organization's List of Essential Medicines. Retinol is available as a generic medication and over ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phylloquinone
Phytomenadione, also known as vitamin K1 or phylloquinone, is a vitamin found in food and used as a dietary supplement. It is on the World Health Organization's List of Essential Medicines. As a supplement it is used to treat certain bleeding disorders. This includes warfarin overdose, vitamin K deficiency, and obstructive jaundice. It is also recommended to prevent and treat vitamin K deficiency bleeding in infants. Use is typically recommended by mouth, intramuscular injection or injection under the skin. When given by injection benefits are seen within two hours. Many countries in the world choose intramuscular injections in newborn to keep them safe from severe bleeding (VKDB). It is considered a safe treatment and saves many children from death and severe neurologic deficit every year. Side effects when given by injection may include pain at the site of injection. Severe allergic reactions may occur when it is injected into a vein or muscle, but this has mainly happened ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
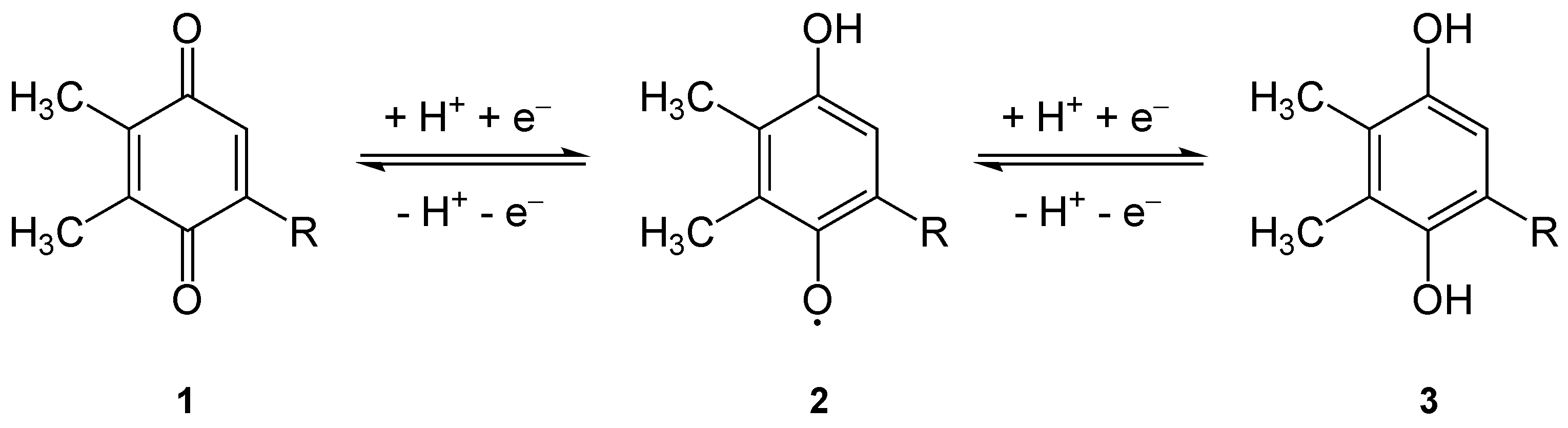
Plastoquinone
Plastoquinone (PQ) is an isoprenoid quinone molecule involved in the electron transport chain in the light-dependent reactions of photosynthesis. The most common form of plastoquinone, known as PQ-A or PQ-9, is a 2,3-dimethyl-1,4-benzoquinone molecule with a side chain of nine isoprenyl units. There are other forms of plastoquinone, such as ones with shorter side chains like PQ-3 (which has 3 isoprenyl side units instead of 9) as well as analogs such as PQ-B, PQ-C, and PQ-D, which differ in their side chains. The benzoquinone and isoprenyl units are both nonpolar, anchoring the molecule within the inner section of a lipid bilayer, where the hydrophobic tails are usually found. Plastoquinones are very structurally similar to ubiquinone, or coenzyme Q10, differing by the length of the isoprenyl side chain, replacement of the methoxy groups with methyl groups, and removal of the methyl group in the 2 position on the quinone. Like ubiquinone, it can come in several oxidation states: p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ubiquinone
Coenzyme Q, also known as ubiquinone and marketed as CoQ10, is a coenzyme family that is ubiquitous in animals and most bacteria (hence the name ubiquinone). In humans, the most common form is coenzyme Q10 or ubiquinone-10. It is a 1,4-benzoquinone, where Q refers to the quinone chemical group and 10 refers to the number of isoprenyl chemical subunits in its tail. In natural ubiquinones, the number can be anywhere from 6 to 10. This family of fat-soluble substances, which resemble vitamins, is present in all respiring eukaryotic cells, primarily in the mitochondria. It is a component of the electron transport chain and participates in aerobic cellular respiration, which generates energy in the form of ATP. Ninety-five percent of the human body's energy is generated this way. Organs with the highest energy requirements—such as the heart, liver, and kidney—have the highest CoQ10 concentrations. There are three redox states of CoQ: fully oxidized (ubiquinone), semiquinone ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
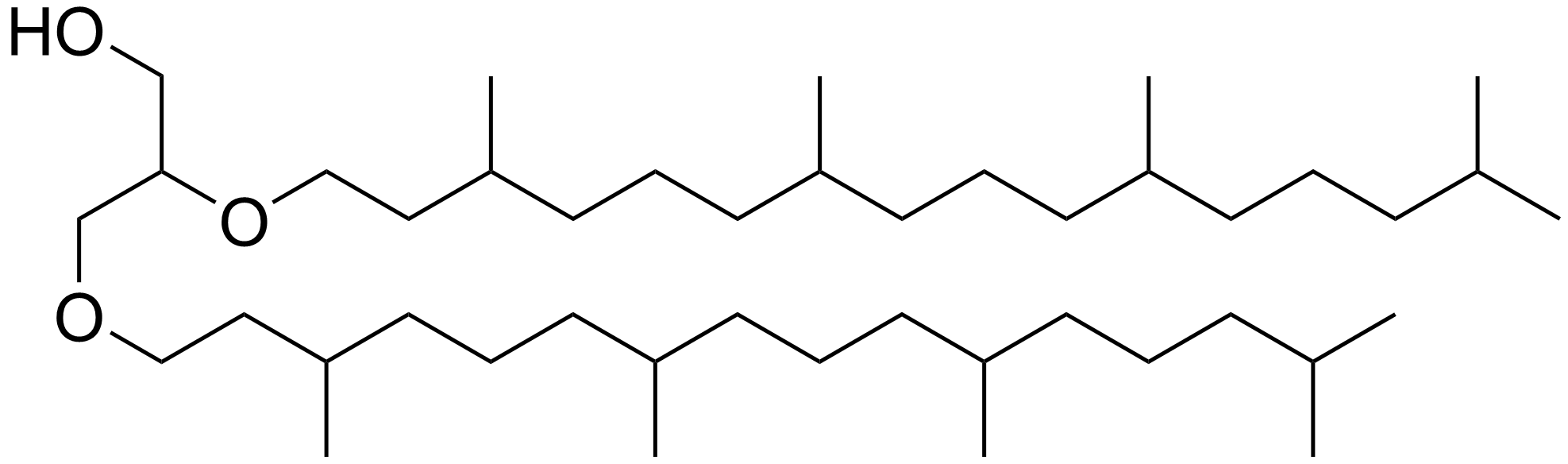
Phytyl
Phytane is the isoprenoid alkane formed when phytol, a constituent of chlorophyll, loses its hydroxyl group. When phytol loses one carbon atom, it yields pristane. Other sources of phytane and pristane have also been proposed than phytol. Pristane and phytane are common constituents in petroleum and have been used as proxies for depositional redox conditions, as well as for correlating oil and its source rock (i.e. elucidating where oil formed). In environmental studies, pristane and phytane are target compounds for investigating oil spills. Chemistry Phytane is a non-polar organic compound that is a clear and colorless liquid at room temperature. It is a head-to-tail linked regular isoprenoid with chemical formula C20H42. Phytane has many structural isomers. Among them, crocetane is a tail-to-tail linked isoprenoid and often co-elutes with phytane during gas chromatography (GC) due to its structural similarity. Phytane also has many stereoisomers because of its three ste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tocopherol
Tocopherols (; TCP) are a class of organic chemical compounds (more precisely, various methylated phenols), many of which have vitamin E activity. Because the vitamin activity was first identified in 1936 from a dietary fertility factor in rats, it was named ''tocopherol'', from Greek τόκος ''tókos'' 'birth' and φέρειν ''phérein'' 'to bear or carry', that is 'to carry a pregnancy', with the ending ''-ol'' signifying its status as a chemical alcohol. α-Tocopherol is the main source found in supplements and in the European diet, where the main dietary sources are olive and sunflower oils, while γ-tocopherol is the most common form in the American diet due to a higher intake of soybean and corn oil. Tocotrienols, which are related compounds, also have vitamin E activity. All of these various derivatives with vitamin activity may correctly be referred to as "vitamin E". Tocopherols and tocotrienols are fat-soluble antioxidants but also seem to have many other func ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phytane
Phytane is the isoprenoid alkane formed when phytol, a constituent of chlorophyll, loses its hydroxyl group. When phytol loses one carbon atom, it yields pristane. Other sources of phytane and pristane have also been proposed than phytol. Pristane and phytane are common constituents in petroleum and have been used as proxies for depositional redox conditions, as well as for correlating oil and its source rock (i.e. elucidating where oil formed). In environmental studies, pristane and phytane are target compounds for investigating oil spills. Chemistry Phytane is a non-polar organic compound that is a clear and colorless liquid at room temperature. It is a head-to-tail linked regular isoprenoid with chemical formula C20H42. Phytane has many structural isomers. Among them, crocetane is a tail-to-tail linked isoprenoid and often co-elutes with phytane during gas chromatography (GC) due to its structural similarity. Phytane also has many stereoisomers because of its three ste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanobacteria
Cyanobacteria (), also known as Cyanophyta, are a phylum of gram-negative bacteria that obtain energy via photosynthesis. The name ''cyanobacteria'' refers to their color (), which similarly forms the basis of cyanobacteria's common name, blue-green algae, although they are not usually scientifically classified as algae. They appear to have originated in a freshwater or terrestrial environment. Sericytochromatia, the proposed name of the paraphyletic and most basal group, is the ancestor of both the non-photosynthetic group Melainabacteria and the photosynthetic cyanobacteria, also called Oxyphotobacteria. Cyanobacteria use photosynthetic pigments, such as carotenoids, phycobilins, and various forms of chlorophyll, which absorb energy from light. Unlike heterotrophic prokaryotes, cyanobacteria have internal membranes. These are flattened sacs called thylakoids where photosynthesis is performed. Phototrophic eukaryotes such as green plants perform photosynthesis in plast ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |