|
Dipeptidases
Dipeptidases are enzymes secreted by enterocytes into the small intestine. Dipeptidases hydrolyze bound pairs of amino acids, called dipeptides. Dipeptidases are secreted onto the brush border of the villi in the small intestine, where they cleave dipeptides into their two component amino acids prior to absorption. They are also found within the enterocytes themselves, performing cytosolic digestion of absorbed dipeptides. Dipeptidases are exopeptidases, classified under EC number 3.4.13. See also * Membrane dipeptidase Membrane dipeptidase (, ''renal dipeptidase'', ''dehydropeptidase I (DPH I)'', ''dipeptidase'', ''aminodipeptidase'', ''dipeptide hydrolase'', ''dipeptidyl hydrolase'', ''nonspecific dipeptidase'', ''glycosyl-phosphatidylinositol-anchored renal di ... References External links * Enzymes EC 3.4.13 {{enzyme-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
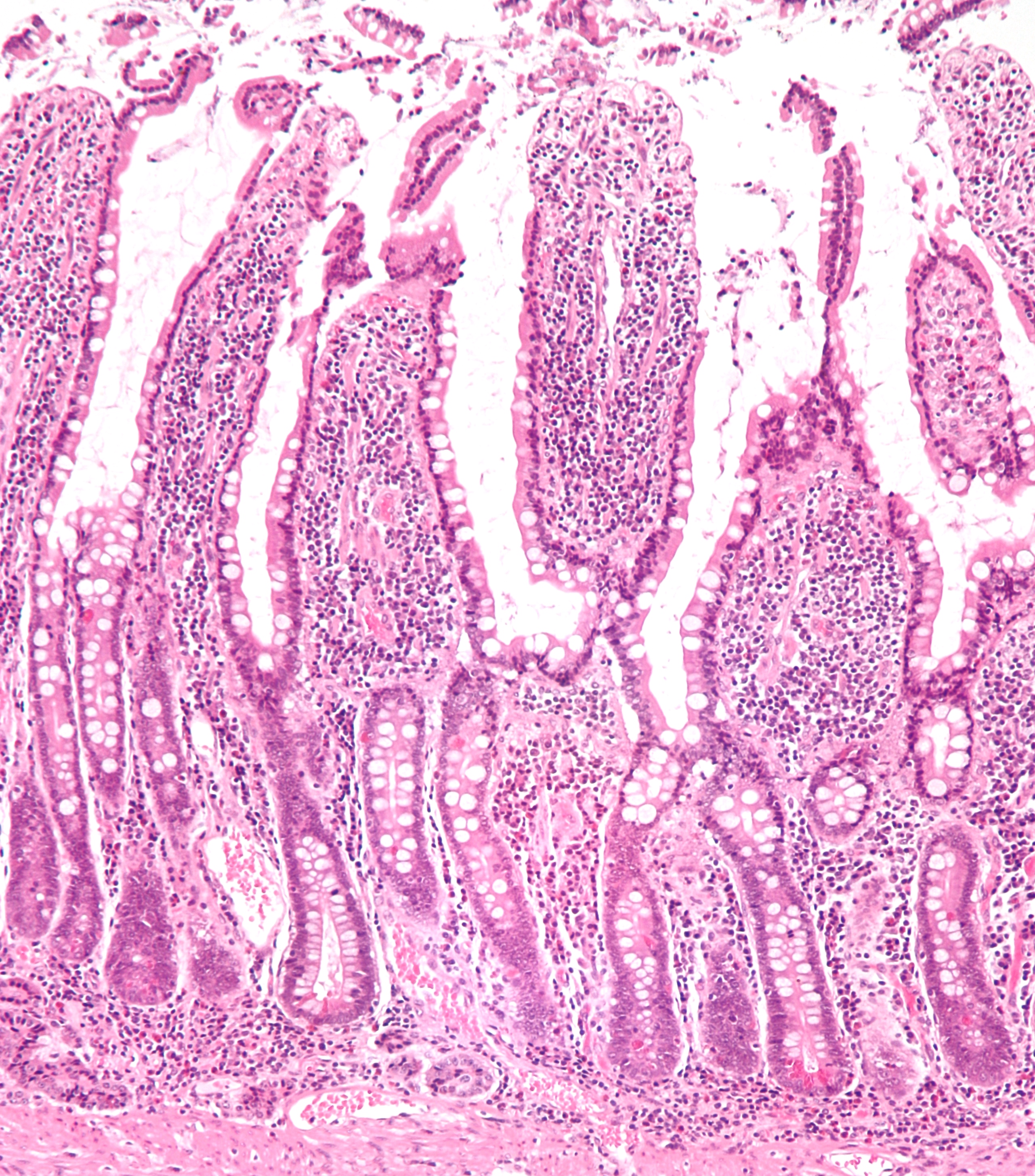
Enterocyte
Enterocytes, or intestinal absorptive cells, are simple columnar epithelial cells which line the inner surface of the small and large intestines. A glycocalyx surface coat contains digestive enzymes. Microvilli on the apical surface increase its surface area. This facilitates transport of numerous small molecules into the enterocyte from the intestinal lumen. These include broken down proteins, fats, and sugars, as well as water, electrolytes, vitamins, and bile salts. Enterocytes also have an endocrine role, secreting hormones such as leptin. Function The major functions of enterocytes include: *Ion uptake, including sodium, calcium, magnesium, iron, zinc, and copper. This typically occurs through active transport. *Water uptake. This follows the osmotic gradient established by Na+/K+ ATPase on the basolateral surface. This can occur transcellularly or paracellularly. *Sugar uptake. Polysaccharides and disaccharidases in the glycocalyx break down large sugar molecules, whi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Small Intestine
The small intestine or small bowel is an organ in the gastrointestinal tract where most of the absorption of nutrients from food takes place. It lies between the stomach and large intestine, and receives bile and pancreatic juice through the pancreatic duct to aid in digestion. The small intestine is about long and folds many times to fit in the abdomen. Although it is longer than the large intestine, it is called the small intestine because it is narrower in diameter. The small intestine has three distinct regions – the duodenum, jejunum, and ileum. The duodenum, the shortest, is where preparation for absorption through small finger-like protrusions called villi begins. The jejunum is specialized for the absorption through its lining by enterocytes: small nutrient particles which have been previously digested by enzymes in the duodenum. The main function of the ileum is to absorb vitamin B12, bile salts, and whatever products of digestion that were not absorbed by th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dipeptide
A dipeptide is an organic compound derived from two amino acids. The constituent amino acids can be the same or different. When different, two isomers of the dipeptide are possible, depending on the sequence. Several dipeptides are physiologically important, and some are both physiologically and commercially significant. A well known dipeptide is aspartame, an artificial sweetener. Dipeptides are white solids. Many are far more water-soluble than the parent amino acids. For example, the dipeptide Ala-Gln has the solubility of 586 g/L more than 10x the solubility of Gln (35 g/L). Dipeptides also can exhibit different stabilities, e.g. with respect to hydrolysis. Gln does not withstand sterilization procedures, whereas this dipeptide does. Because dipeptides are prone to hydrolysis, the high solubility is exploited in infusions, i.e. to provide nutrition. Examples Commercial value About six dipeptides are of commercial interest. *Aspartame (''N''-L-α-aspartyl-L-phenyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brush Border
A brush border (striated border or brush border membrane) is the microvilli-covered surface of simple cuboidal and simple columnar epithelium found in different parts of the body. Microvilli are approximately 100 nanometers in diameter and their length varies from approximately 100 to 2,000 nanometers. Because individual microvilli are so small and are tightly packed in the brush border, individual microvilli can only be resolved using electron microscopes; with a light microscope they can usually only be seen collectively as a fuzzy fringe at the surface of the epithelium. This fuzzy appearance gave rise to the term brush border, as early anatomists noted that this structure appeared very much like the bristles of a paintbrush. Brush border cells are found mainly in the following organs: * The small intestine tract: This is where absorption takes place. The brush borders of the intestinal lining are the site of terminal carbohydrate digestions. The microvilli that constitut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exopeptidase
An exopeptidase is any peptidase that catalyzes the cleavage of the terminal (or the penultimate) peptide bond; the process releases a single amino acid, dipeptide or a tripeptide from the peptide chain. Depending on whether the amino acid is released from the amino or the carboxy terminal (N-terminus or C-terminus), an exopeptidase is further classified as an aminopeptidase or a carboxypeptidase, respectively. Thus, an aminopeptidase, an enzyme in the brush border of the small intestine, will cleave a single amino acid from the amino terminal, whereas carboxypeptidase, which is a digestive enzyme present in pancreatic juice, will cleave a single amino acid from the carboxylic end of the peptide. Some examples of exopeptidases include: * Carboxypeptidase A - cleaves C-terminal Phe, Tyr, Trp, or Leu * Carboxypeptidase B - cleaves C-terminal Lys or Arg * Aminopeptidase - cleaves any N-terminal amino acid * Prolinase - cleaves N-terminal Pro from dipeptides * Prolidase - cleaves ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme Commission Number
The Enzyme Commission number (EC number) is a numerical classification scheme for enzymes, based on the chemical reactions they catalyze. As a system of enzyme nomenclature, every EC number is associated with a recommended name for the corresponding enzyme-catalyzed reaction. EC numbers do not specify enzymes but enzyme-catalyzed reactions. If different enzymes (for instance from different organisms) catalyze the same reaction, then they receive the same EC number. Furthermore, through convergent evolution, completely different protein folds can catalyze an identical reaction (these are sometimes called non-homologous isofunctional enzymes) and therefore would be assigned the same EC number. By contrast, UniProt identifiers uniquely specify a protein by its amino acid sequence. Format of number Every enzyme code consists of the letters "EC" followed by four numbers separated by periods. Those numbers represent a progressively finer classification of the enzyme. Preliminary EC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Membrane Dipeptidase
Membrane dipeptidase (, ''renal dipeptidase'', ''dehydropeptidase I (DPH I)'', ''dipeptidase'', ''aminodipeptidase'', ''dipeptide hydrolase'', ''dipeptidyl hydrolase'', ''nonspecific dipeptidase'', ''glycosyl-phosphatidylinositol-anchored renal dipeptidase'', ''MBD'', ''MDP'', ''leukotriene D4 hydrolase'') is an enzyme. This enzyme catalyses the following chemical reaction : Hydrolysis of dipeptides (e.g., leukotriene D4, cystinyl-bis-glycine, some β-lactam antibiotics (e.g., carbapenem)) This membrane-bound, zinc enzyme has broad specificity. Inhibitors include bestatin and cilastatin Cilastatin inhibits the human enzyme dehydropeptidase. Uses Dehydropeptidase is an enzyme found in the kidney and is responsible for degrading the antibiotic imipenem. Cilastatin can therefore be combined intravenously with imipenem in order .... Genes * Dipeptidase 1 (DPEP1) * Dipeptidase 2 (DPEP2) * Dipeptidase 3 (DPEP3) References External links * {{Portal bar, Bio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzymes
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |