|
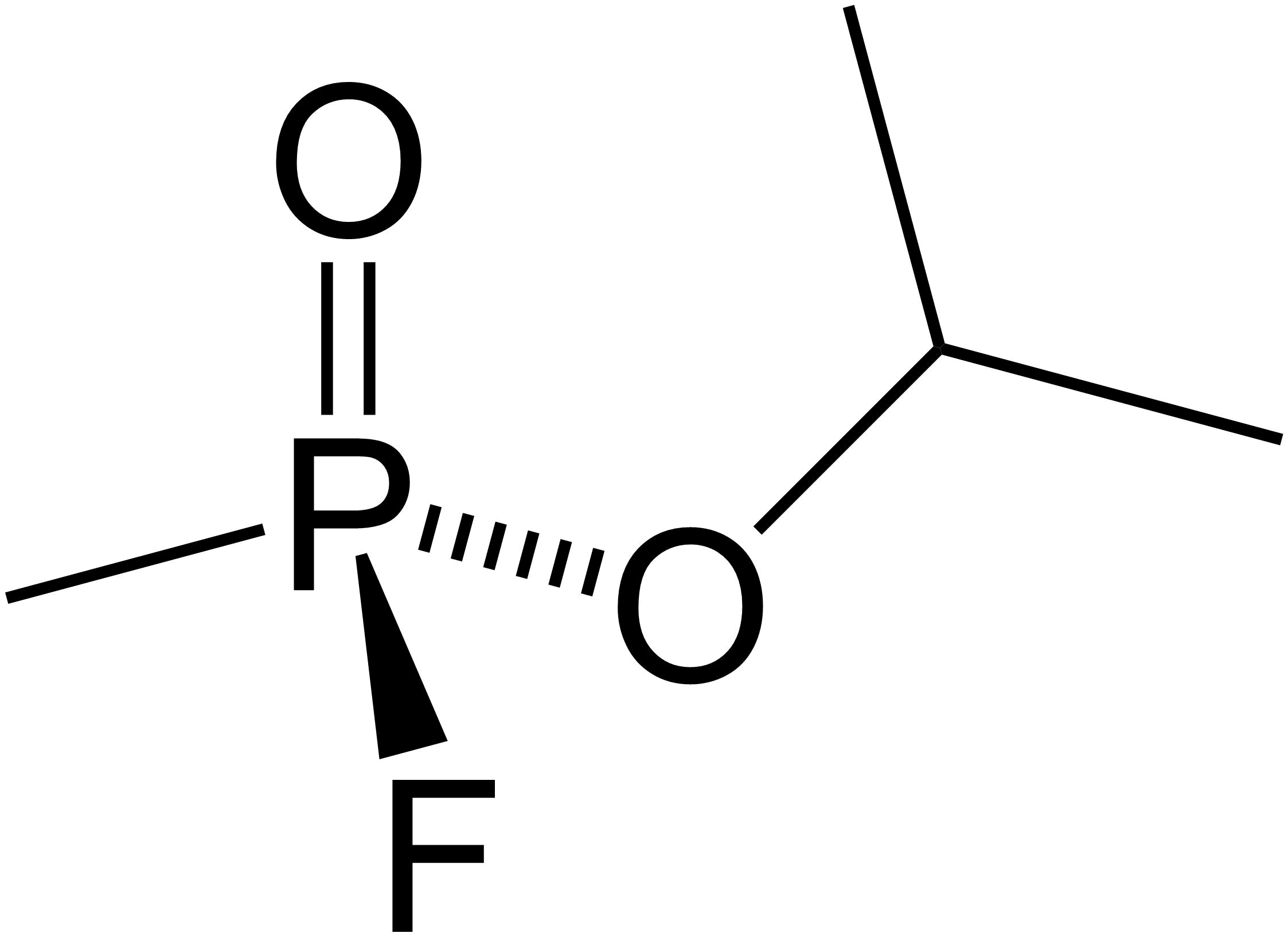
Diethyl Chlorophosphate
Diethyl chlorophosphate is an organophosphorus compound with the formula (C2H5O)2P(O)Cl. As an reagent in organic synthesis, it is use the convert alcohols to the corresponding diethylphosphate esters. It is a colorless liquid with a fruity odor. It is a corrosive, and as a cholinesterase inhibitor, highly toxic through dermal absorption. The molecule is tetrahedral. Synthesis and reactions The compound is prepared by the chlorination of diethylphosphite with carbon tetrachloride (Atherton–Todd reaction). The compound is electrophilic. Controlled hydrolysis gives tetraethyl pyrophosphate Tetraethyl pyrophosphate, abbreviated TEPP, is an organophosphate compound with the formula . It is the tetraEthyl group, ethyl derivative of pyrophosphate (P2O74-). It is a colorless oil that solidifies near room temperature. It is used as an ins .... Alcohols react to vie mixed phosphate esters: :(C2H5O)2P(O)Cl + ROH → (C2H5O)2P(O)OR + HCl The reagent is routinely employed in or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphorus Compound
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents. Organophosphorus chemistry is the corresponding science of the properties and reactivity of organophosphorus compounds. Phosphorus, like nitrogen, is in group 15 of the periodic table, and thus phosphorus compounds and nitrogen compounds have many similar properties. The definition of organophosphorus compounds is variable, which can lead to confusion. In industrial and environmental chemistry, an organophosphorus compound need contain only an organic substituent, but need not have a direct phosphorus-carbon (P-C) bond. Thus a large proportion of pesticides (e.g., malathion), are often included in this class of compounds. Phosphorus can adopt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: ''total synthesis'', ''semisynthesis'', and ''methodology''. Total synthesis A total synthesis is the complete chemical synthesis of complex organic molecules from simple, commercially available petrochemical or natural precursors. Total synthesis may be accomplished either via a linear or convergent approach. In a ''linear'' synthesis—often adequate for simple structures—several steps are performed one after another until the molecule is complete; the chemical compounds made in each step are called synthetic intermediates. Most often, each step in a synthesis refers to a separate rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol (chemistry)
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest examples, includes all compounds which conform to the general formula . Simple monoalcohols that are the subject of this article include primary (), secondary () and tertiary () alcohols. The suffix ''-ol'' appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority. When a higher priority group is present in the compound, the prefix ''hydroxy-'' is used in its IUPAC name. The suffix ''-ol'' in non-IUPAC names (such as paracetamol or cholesterol) also typically indicates that the substance is an alcohol. However, some compou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphate Ester
In organic chemistry, organophosphates (also known as phosphate esters, or OPEs) are a class of organophosphorus compounds with the general structure , a central phosphate molecule with alkyl or Aryl, aromatic substituents. They can be considered as esters of phosphoric acid. Like most functional groups, organophosphates occur in a diverse range of forms, with important examples including key biomolecules such as DNA, RNA and adenosine triphosphate, ATP, as well as many insecticides, herbicides, nerve agents and flame retardants. OPEs have been widely used in various products as flame retardants, plasticizers, and performance additives to engine oil. The popularity of OPEs as flame retardants came as a substitution for the highly regulated brominated flame retardants. The low cost of production and compatibility to diverse polymers made OPEs to be widely used in industry including textile, furniture, electronics as plasticizers and flame retardants. These compounds are added t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cholinesterase Inhibitors
Cholinesterase inhibitors (ChEIs), also known as anti-cholinesterase, are chemicals that prevent the breakdown of the neurotransmitter acetylcholine or butyrylcholine. This increases the amount of the acetylcholine or butyrylcholine in the synaptic cleft that can bind to muscarinic receptors, nicotinic receptors and others. This group of inhibitors is divided into two subgroups, acetylcholinesterase inhibitors (AChEIs) and butyrylcholinesterase inhibitors (BChEIs). ChEIs may be used as drugs for Alzheimer's disease, Alzheimer's and myasthenia gravis, and also as chemical weapons and insecticides. Side effects when used as drugs may include loss of appetite, nausea, vomiting, loose stools, vivid dreams at night, dehydration, rash, bradycardia, peptic ulcer disease, seizures, weight loss, rhinorrhea, salivation, muscle cramps, and fasciculations. ChEIs are indirect-acting parasympathomimetic drugs. ChEls are widely used as chemical weapons. Since November 2019 the group of AChe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethylphosphite
Diethylphosphite is the organophosphorus compound with the formula (C2H5O)2P(O)H. It is a popular reagent for generating other organophosphorus compounds, exploiting the high reactivity of the P-H bond. Diethylphosphite is a colorless liquid. The molecule is tetrahedral. Synthesis and properties The compound was probably prepared in the 1850s by combining phosphorus trichloride and ethanol, but intentional preparations came later. It arises as follows: :PCl3 + 3 C2H5OH → (C2H5O)2P(O)H + 2 HCl + C2H5Cl Many derivatives can be prepared similarly. Despite being named as a phosphite it exists overwhelmingly in its phosphonate form, , a property it shares with its parent acid phosphorous acid; despite this many of its reactions are difficult to rationalise without assuming the existence of the following tautomerism equilibrium between phosphorus(V) and phosphorus(III) forms: :(C2H5O)2PV(O)H ⇌ (C2H5O)2PIII(OH) Reactions Alkoxide exchange Diethylphosphite undergoes transesterific ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Tetrachloride
Carbon tetrachloride, also known by many other names (such as tetrachloromethane, also IUPAC nomenclature of inorganic chemistry, recognised by the IUPAC, carbon tet in the cleaning industry, Halon-104 in firefighting, and Refrigerant-10 in HVACR) is an organic compound with the chemical formula CCl4. It is a colourless liquid with a "sweet" smell that can be detected at low levels. It is practically incombustible at lower temperatures. It was formerly widely used in fire extinguishers, as a precursor to refrigerants and as a cleaning agent, but has since been phased out because of environmental and safety concerns. Exposure to high concentrations of carbon tetrachloride (including vapor) can affect the central nervous system and degenerate the liver and kidneys. Prolonged exposure can be fatal. Properties In the carbon tetrachloride molecule, four chlorine atoms are positioned symmetrically as corners in a tetrahedron, tetrahedral configuration joined to a central carbon atom by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atherton–Todd Reaction
The Atherton-Todd reaction is a name reaction in organic chemistry, which goes back to the British chemists F. R. Atherton, H. T. Openshaw and A. R. Todd. These described the reaction for the first time in 1945 as a method of converting Dialkyl phosphite, dialkyl phosphites into dialkyl chlorophosphates. The dialkyl chlorophosphates formed are often too reactive to be isolated, though. For this reason, the synthesis of Phosphate, phosphates or Phosphoramidate, phosphoramidates can follow the Atherton-Todd reaction in the presence of alcohol (chemistry), alcohols or Amine, amines. The following equation gives an overview over the Atherton-Todd reaction using the Reagent, reactant dimethyl phosphite as an example: The reaction takes place after the addition of tetrachloromethane and a Base (chemistry), base. This base is usually a primary, secondary or tertiary amine. Instead of methyl groups other Alkyl group, alkyl or Benzyl group, benzyl groups may be present. Reaction mechanis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carries a partial positive charge, or have an atom that does not have an octet of electrons. Electrophiles mainly interact with nucleophiles through addition and substitution reactions. Frequently seen electrophiles in organic syntheses include cations such as H+ and NO+, polarized neutral molecules such as HCl, alkyl halides, acyl halides, and carbonyl compounds, polarizable neutral molecules such as Cl2 and Br2, oxidizing agents such as organic peracids, chemical species that do not satisfy the octet rule such as carbenes and radicals, and some Lewis acids such as BH3 and DIBAL. Organic chemistry Addition of halogens These occur between alkenes and electrophiles, often halogens as in halogen addition reactions. Common reaction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetraethyl Pyrophosphate
Tetraethyl pyrophosphate, abbreviated TEPP, is an organophosphate compound with the formula . It is the tetraEthyl group, ethyl derivative of pyrophosphate (P2O74-). It is a colorless oil that solidifies near room temperature. It is used as an insecticide. The compound hydrolyzes rapidly. Applications TEPP is an insecticide to aphids, mites, spiders, mealybugs, leafhoppers, lygus bugs, thrips, leafminers, and many other pests. TEPP and other organophosphates are the most widely used pesticides in the U.S. due to their effectiveness and relative small impact on the environment because this organophosphate breaks down so easily. TEPP has been used for treatment for myasthenia gravis, an autoimmune disease. The treatment would deliver an increase in strength. Synthesis The synthesis by De Clermont and Moschnin was based on the earlier work by Alexander Williamson (who is well known for the Williamson ether synthesis, Williamson-synthesis of ethers). Their synthesis made use of eth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |