|
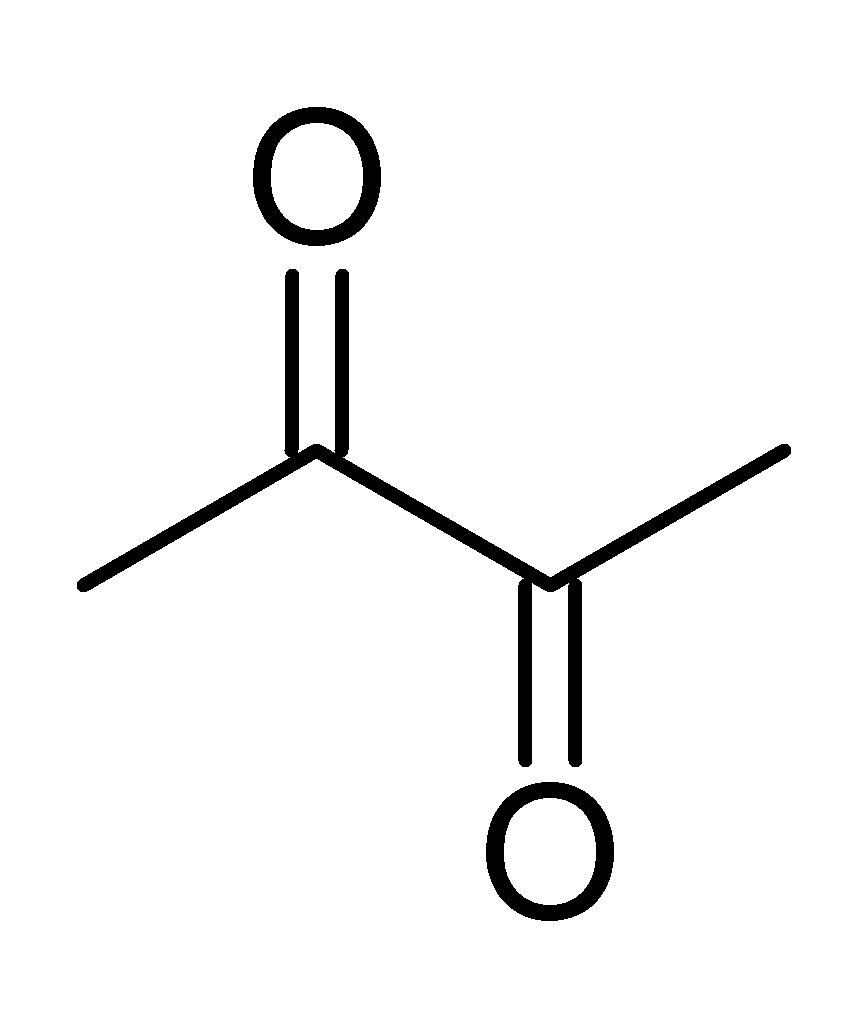
Diacetyl
Diacetyl (IUPAC systematic name: butanedione or butane-2,3-dione) is an organic compound with the chemical formula (CH3CO)2. It is a yellow liquid with an intensely buttery flavor. It is a vicinal diketone (two C=O groups, side-by-side). Diacetyl occurs naturally in alcoholic beverages and is added as a flavoring to some foods to impart its buttery flavor. Chemical structure A distinctive feature of diacetyl (and other vicinal diketones) is the long C–C bond linking the carbonyl centers. This bond distance is about 1.54 Å, compared to 1.45 Å for the corresponding C–C bond in 1,3-butadiene. The elongation is attributed to repulsion between the polarized carbonyl carbon centers. Occurrence and biosynthesis Diacetyl arises naturally as a byproduct of fermentation. In some fermentative bacteria, it is formed via the thiamine pyrophosphate-mediated condensation of pyruvate and acetyl CoA. Sour (cultured) cream, cultured buttermilk, and cultured butter are produced by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Artificial Butter Flavoring
Artificial butter flavoring is a flavoring used to give a food the taste and smell of butter. It may contain diacetyl, acetylpropionyl, or acetoin, three natural compounds in butter that contribute to its characteristic taste and smell. Manufacturers of margarines or similar oil-based products typically add it (along with beta carotene for the yellow color) to make the final product butter-flavored, because it would otherwise be relatively tasteless. Butter-flavoring controversy The lung disease bronchiolitis obliterans is attributed to prolonged exposure to diacetyl, e.g. in an industrial setting. Workers in several factories that manufacture artificial butter flavoring have been diagnosed with bronchiolitis obliterans, a rare and serious disease of the lungs. The disease has been called "popcorn worker's lung" or "popcorn lung" because it was first seen in former workers of a microwave popcorn factory in Missouri, but NIOSH refers to it by the more general term "flavori ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bronchiolitis Obliterans
Bronchiolitis obliterans (BO), also known as obliterative bronchiolitis, constrictive bronchiolitis and popcorn lung, is a disease that results in obstruction of the smallest airways of the lungs (bronchioles) due to inflammation. Symptoms include a dry cough, shortness of breath, wheezing and feeling tired. These symptoms generally get worse over weeks to months. It is not related to cryptogenic organizing pneumonia, previously known as bronchiolitis obliterans organizing pneumonia. Causes include breathing in toxic fumes, respiratory infections, Connective tissue disease, connective tissue disorder or complications following a Hematopoietic stem cell transplantation, bone marrow or Heart–lung transplant, heart-lung transplant. Symptoms may not occur until two to eight weeks following toxic exposure or infection. The underlying mechanism involves inflammation that results in Scar, scar tissue formation. Diagnosis is by CT scan, Pulmonary function testing, pulmonary function te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetoin
Acetoin, also known as 3-hydroxybutanone or acetyl methyl carbinol, is an organic compound with the formula CH3CH(OH)C(O)CH3. It is a colorless liquid with a pleasant, buttery odor. It is chiral. The form produced by bacteria is (''R'')-acetoin.Albert Gossauer: ''Struktur und Reaktivität der Biomoleküle'', Verlag Helvetica Chimica Acta, Zürich, 2006, Seite 285, . Production in bacteria Acetoin is a neutral, four-carbon molecule used as an external energy store by a number of fermentative bacteria. It is produced by the decarboxylation of alpha-acetolactate, a common precursor in the biosynthesis of branched-chain amino acids. Owing to its neutral nature, production and excretion of acetoin during exponential growth prevents over-acidification of the cytoplasm and the surrounding medium that would result from accumulation of acidic metabolic products, such as acetic acid and citric acid. Once superior carbon sources are exhausted, and the culture enters stationary phase, ace ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diketone
In organic chemistry, a dicarbonyl is a molecule containing two carbonyl () groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls are in close enough proximity that their reactivity is changed, such as 1,2-, 1,3-, and 1,4-dicarbonyls. Their properties often differ from those of monocarbonyls, and so they are usually considered functional groups of their own. These compounds can have symmetrical or unsymmetrical substituents on each carbonyl, and may also be functionally symmetrical (dialdehydes, diketones, diesters, ''etc.'') or unsymmetrical (keto-esters, keto-acids, ''etc.''). 1,2-Dicarbonyls 1,2-Dialdehyde The only 1,2-dialdehyde is glyoxal, . Like many alkyldialdehydes, glyoxal is encountered almost exclusively as its hydrate and oligomers thereof. These derivatives often behave equivalently to the aldehydes since hydration is reversible. Glyoxal condenses re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetoin
Acetoin, also known as 3-hydroxybutanone or acetyl methyl carbinol, is an organic compound with the formula CH3CH(OH)C(O)CH3. It is a colorless liquid with a pleasant, buttery odor. It is chiral. The form produced by bacteria is (''R'')-acetoin.Albert Gossauer: ''Struktur und Reaktivität der Biomoleküle'', Verlag Helvetica Chimica Acta, Zürich, 2006, Seite 285, . Production in bacteria Acetoin is a neutral, four-carbon molecule used as an external energy store by a number of fermentative bacteria. It is produced by the decarboxylation of alpha-acetolactate, a common precursor in the biosynthesis of branched-chain amino acids. Owing to its neutral nature, production and excretion of acetoin during exponential growth prevents over-acidification of the cytoplasm and the surrounding medium that would result from accumulation of acidic metabolic products, such as acetic acid and citric acid. Once superior carbon sources are exhausted, and the culture enters stationary phase, ace ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butter
Butter is a dairy product made from the fat and protein components of churned cream. It is a semi-solid emulsion at room temperature, consisting of approximately 80% butterfat. It is used at room temperature as a spread, melted as a condiment, and used as a fat in baking, sauce-making, pan frying, and other cooking procedures. Most frequently made from cow's milk, butter can also be manufactured from the milk of other mammals, including sheep, goats, buffalo, and yaks. It is made by churning milk or cream to separate the fat globules from the buttermilk. Salt has been added to butter since antiquity to help to preserve it, particularly when being transported; salt may still play a preservation role but is less important today as the entire supply chain is usually refrigerated. In modern times salt may be added for its taste. Food colorings are sometimes added to butter. Rendering butter, removing the water and milk solids, produces clarified butter or ''ghee'', which is a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Construction Of Electronic Cigarettes
An electronic cigarette is a handheld battery (electricity), battery-powered Vaporizer (inhalation device), vaporizer that simulates tobacco smoking, smoking, but without tobacco combustion. E-cigarette components include a mouthpiece (drip tip), a cartridge (liquid storage area), a heating element/atomizer nozzle, atomizer, a microprocessor, a battery, and some of them have an LED lamp, LED light on the end. An atomizer consists of a small heating element, or coil, that vaporizes e-liquid and a capillary action, wicking material that draws liquid onto the coil. When the user inhales a flow sensor activates the heating element that atomizes the Solution (chemistry)#Liquid solutions, liquid solution; most devices are manually activated by a push-button. The e-liquid reaches a temperature of roughly within a chamber to create an aerosolized vapor. The user inhales an aerosol, which is commonly but inaccurately called vapor, rather than cigarette smoke. Vaping is different from smo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chardonnay
Chardonnay (, , ) is a green-skinned grape variety used in the production of white wine. The variety originated in the Burgundy wine region of eastern French wine, France, but is now grown wherever wine is produced, from English wine, England to New Zealand wine, New Zealand. For new and developing wine regions, growing Chardonnay is seen as a 'rite of passage' and an easy entry into the international wine market. The Chardonnay grape itself is neutral, with many of the flavors commonly associated with the wine being derived from such influences as ''terroir'' and oak (wine), oak.Robinson, 2006, pp. 154–56. It is vinified in many different styles, from the lean, crisply mineral wines of Chablis, France, to New World wines with oak and tropical fruit flavors. In cool climates (such as Chablis and the Carneros AVA of California (wine), California), Chardonnay wine tends to be medium to light body with noticeable acidity (wine), acidity and flavors of green plum, apple, and pe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carboxylation, the addition of CO2 to a compound. Enzymes that catalyze decarboxylations are called decarboxylases or, the more formal term, carboxy-lyases (Enzyme Commission number, EC number 4.1.1). In organic chemistry The term "decarboxylation" usually means replacement of a carboxyl group () with a hydrogen atom: :RCO2H -> RH + CO2 Decarboxylation is one of the oldest known organic reactions. It is one of the processes assumed to accompany pyrolysis and destructive distillation. Metal salts, especially copper compounds, facilitate the reaction via the intermediacy of metal carboxylate complexes. Decarboxylation of aryl carboxylates can generate the equivalent of the correspond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetolactate
α-Acetolactic acid is a precursor in the biosynthesis of the branched chain amino acids valine and leucine. α-Acetolactic acid is produced from two molecules of pyruvic acid by acetolactate synthase. α-Acetolactic acid can also be decarboxylated by alpha-acetolactate decarboxylase to produce acetoin. The name α-acetolactate is used for anion (conjugate base), salts, and ester In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...s of α-acetolactic acid. References {{Reflist Alpha hydroxy acids Beta-keto acids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valine
Valine (symbol Val or V) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α- carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain isopropyl group, making it a non-polar aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Human dietary sources are foods that contain protein, such as meats, dairy products, soy products, beans and legumes. It is encoded by all codons starting with GU (GUU, GUC, GUA, and GUG). History and etymology Valine was first isolated from casein in 1901 by Hermann Emil Fischer. The name valine comes from valeric acid, which in turn is named after the plant valerian due to the presence of the acid in the roots of the plant. Nomenclature According to IUPAC, carbon atoms forming valine are numbered sequentially s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |