|
Depolarizer
A depolarizer or depolariser, in electrochemistry, according to an IUPAC definition, is a synonym of electroactive substance, i.e., a substance which changes its oxidation state, or partakes in a formation or breaking of chemical bonds, in a charge-transfer step of an electrochemical reaction. In the battery industry, the term "depolarizer" has been used to denote a substance used in a primary cell to prevent buildup of hydrogen gas bubbles."McGraw-Hill Dictionary of Scientific & Technical Terms", McGraw-Hill, Inc., 2003. A battery depolarizer takes up electrons during discharge of the cell; therefore, it is always an oxidizing agent. The term "depolarizer" can be considered as outdated or misleading, since it is based on the concept of " polarization" which is hardly realistic in many cases. Polarization Under certain conditions for some electrochemical cells, especially if they use an aqueous electrolyte, hydrogen ions can be converted into hydrogen atoms and H2 molecules. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
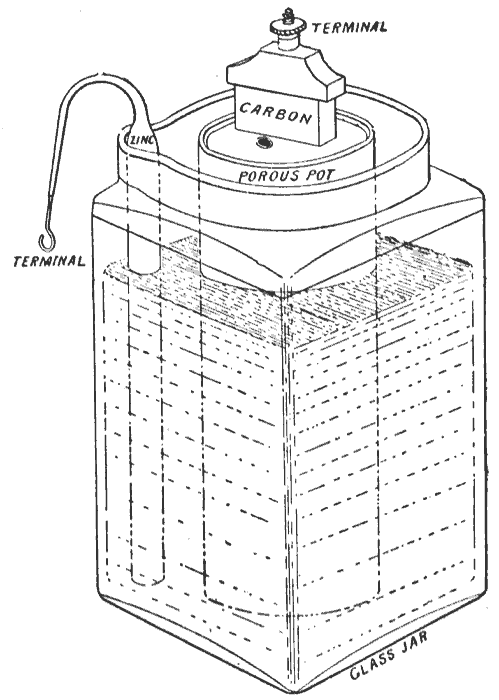
Leclanché Cell
The Leclanché cell is a battery invented and patented by the French scientist Georges Leclanché in 1866. The battery contained a conducting solution (electrolyte) of ammonium chloride, a cathode (positive terminal) of carbon, a depolarizer of manganese dioxide (oxidizer), and an anode (negative terminal) of zinc (reductant). The chemistry of this cell was later successfully adapted to manufacture a dry cell. History In 1866, Georges Leclanché invented a battery that consisted of a zinc anode and a manganese dioxide cathode wrapped in a porous material, dipped in a jar of ammonium chloride solution. The manganese dioxide cathode had a little carbon mixed into it as well, which improved conductivity and absorption. It provided a voltage of 1.4 volts.Simms, J.W. (1965) ''The Boy Electrician'' M.I.E.E. p. 61 This cell achieved very quick success in telegraphy, signalling and electric bell work. The dry cell form was used to power early telephones—usually from an adjacent woo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromic Acid Cell
The Chromic acid cell was a type of primary cell which used chromic acid as a depolarizer. The chromic acid was usually made by acidifying (with sulfuric acid) a solution of potassium dichromate. The old name for potassium dichromate was potassium bichromate and the cell was often called a Bichromate cell.Ayrton, W.E. and Mather, T. ''Practical Electricity'', Cassell and Company, London, 1911, pp 185-187 This type of cell is now only of historical interest. History Construction The main elements of the cell were: * Anode, zinc * Electrolyte, dilute sulfuric acid * Depolarizer, chromic acid * Cathode, carbon The cell was made in two forms - the single-fluid type, attributed to Poggendorff and the two-fluid type, attributed to Fuller. In both cases, cell voltage was about 2 volts. Poggendorff cell The cell was set up in a long-necked glass bottle with a zinc plate located between two carbon plates. The electrolyte and depolarizer were mixed. The mixture would dissolve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clark Cell
The Clark cell, invented by English engineer Josiah Latimer Clark in 1873, is a wet-chemical cell (colloquially: ''battery'') that produces a highly stable voltage. In 1893, the output of the Clark cell at 15 °C was defined by the International Electrical Congress as 1.434 volts, and this definition became law in the United States in 1894. This definition was later supplanted by one based on the Weston cell. Chemistry Clark cells use a zinc, or zinc amalgam, anode and a mercury cathode in a saturated aqueous solution of zinc sulfate, with a paste of mercurous sulfate as depolarizer. Construction Original cell Clark's original cell was set up in a glass jar in a similar way to a gravity Daniell cell. The copper cathode was replaced by a pool of mercury at the bottom of the jar. Above this was the mercurous sulfate paste and, above that, the zinc sulfate solution. A short zinc rod dipped into the zinc sulfate solution. The zinc rod was supported by a cork with two holes � ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Weston Cell
The Weston standard cell is a wet-chemical cell that produces a highly stable voltage suitable as a laboratory standard for calibration In measurement technology and metrology, calibration is the comparison of measurement values delivered by a device under test with those of a calibration standard of known accuracy. Such a standard could be another measurement device of know ... of voltmeters. Invented by Edward Weston (chemist), Edward Weston in 1893, it was adopted as the International Standard for electromotive force, EMF from 1911 until superseded by the Josephson voltage standard in 1990. Chemistry The anode is an amalgam (chemistry), amalgam of cadmium with mercury (element), mercury with a cathode of pure mercury over which a paste of mercurous sulfate and mercury is placed. The electrolyte is a saturated solution of cadmium sulfate, and the depolarizer is a paste of mercurous sulfate. As shown in the illustration, the cell is set up in an H-shaped glass vessel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polarisation (electrochemistry)
In electrochemistry, polarization is a collective term for certain mechanical side-effects (of an electrochemical process) by which isolating barriers develop at the interface between electrode and electrolyte. These side-effects influence the reaction mechanisms, as well as the chemical kinetics of corrosion and metal deposition.. In a reaction we can displace the bonding electrons by attacking reagents. The electronic displacement in turn may be due to certain effects, some of which are permanent (inductive and mesomeric effects), and the others are temporary (electromeric effect). Those effects which are permanently operating in the molecule are known as polarization effects, and those effects which are brought into play by attacking reagent (and as the attacking reagent is removed, the electronic displacement disappears) are known as polarisability effects. The term 'polarization' derives from the early 19th-century discovery that electrolysis causes the elements in an electroly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. This administrative office is headed by IUPAC's executive director, currently Lynn Soby. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national chemistry societies, national academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organizations. IUPAC's Inter-divisional Committee on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Everyman's Encyclopaedia
''Everyman's Encyclopaedia'' is an encyclopedia published by Joseph Dent from 1913 as part of the Everyman's Library. The set was descended from the 1850s ''English Cyclopaedia'' of 1854, which in turn was based on the ''Penny Cyclopaedia'' of the 1830s. Originally published in 1913–14 by J.M. Dent in London and, simultaneously, E. P. Dutton in New York, the encyclopedia proved popular due to its low cost, small size and concise but highly accurate articles. The set did, however, lack much illustrative material. Andrew Boyle was credited as the editor. A second edition was published in 1931–32. The title was slightly different, changing from ''The Everyman Encyclopedia'' to ''Everyman's Encyclopaedia''. This set had 12 volumes, 7 million words, 9,000 pages and 50,000 articles. There was also an optional atlas volume. Athelstan Ridgway was credited as the editor. The third edition was published in 1950, again under Ridgways direction, and published in London by Dent, but no ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury(I) Sulfate
Mercury(I) sulfate, commonly called mercurous sulphate ( UK) or mercurous sulfate ( US) is the chemical compound Hg2SO4. Mercury(I) sulfate is a metallic compound that is a white, pale yellow or beige powder. It is a metallic salt of sulfuric acid formed by replacing both hydrogen atoms with mercury(I). It is highly toxic; it could be fatal if inhaled, ingested, or absorbed by skin. Structure In the crystal, mercurous sulfate is made up of Hg22+ center with an Hg-Hg distance of about 2.50 Å. The SO42− anions form both long and short Hg-O bonds ranging from 2.23 to 2.93 Å. Focusing on the shorter Hg-O bonds, the Hg – Hg – O bond angle is 165°±1°. Preparation One way to prepare mercury(I) sulfate is to mix the acidic solution of mercury(I) nitrate with 1 to 6 sulfuric acid solution:, [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Daniell Cell
The Daniell cell is a type of electrochemical cell invented in 1836 by John Frederic Daniell, a British chemist and meteorologist, and consists of a copper pot filled with a copper (II) sulfate solution, in which is immersed an unglazed earthenware container filled with sulfuric acid and a zinc electrode. He was searching for a way to eliminate the hydrogen bubble problem found in the voltaic pile, and his solution was to use a second electrolyte to consume the hydrogen produced by the first. Zinc sulfate may be substituted for the sulfuric acid. The Daniell cell was a great improvement over the existing technology used in the early days of battery development. A later variant of the Daniell cell called the gravity cell or crowfoot cell was invented in the 1860s by a Frenchman named Callaud and became a popular choice for electrical telegraphy. The Daniell cell is also the historical basis for the contemporary definition of the volt, which is the unit of electromotive force in t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper(II) Sulfate
Copper(II) sulfate, also known as copper sulphate, is an inorganic compound with the chemical formula . It forms hydrates , where ''n'' can range from 1 to 7. The pentahydrate (''n'' = 5), a bright blue crystal, is the most commonly encountered hydrate of copper(II) sulfate. Older names for the pentahydrate include blue vitriol, bluestone, vitriol of copper,Antoine-François de Fourcroy, tr. by Robert Heron (1796) "Elements of Chemistry, and Natural History: To which is Prefixed the Philosophy of Chemistry". J. Murray and others, Edinburgh. Page 348. and Roman vitriol.Oxford University Press,Roman vitriol, Oxford Living Dictionaries. Accessed on 2016-11-13 It exothermically dissolves in water to give the aquo complex , which has octahedral molecular geometry. The structure of the solid pentahydrate reveals a polymeric structure wherein copper is again octahedral but bound to four water ligands. The centers are interconnected by sulfate anions to form chains. Anhydrous copper sulfa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver-oxide Battery
A silver-oxide battery (IEC code: S) is a primary cell using silver oxide as the cathode material and zinc for the anode. These cells maintain a nearly constant nominal voltage during discharge until fully depleted. They are available in small sizes as button cells, where the amount of silver used is minimal and not a prohibitively expensive contributor to the overall product cost. Silver-oxide primary batteries account for 30% of all primary battery sales in Japan (64 out of 212 million in February 2020). Silver-oxide batteries were used on Apollo program lunar missions for the lunar module and lunar rover power supplies because of their high energy-to-weight ratio.Lyons, Pete; "10 Best Ahead-of-Their-Time Machines", ''Car and Driver'', Jan. 1988, p.78 Chemistry A silver-oxide battery uses silver(I) oxide as the positive electrode (cathode), zinc as the negative electrode (anode), plus an alkaline electrolyte, usually sodium hydroxide (NaOH) or potassium hydroxide (KOH). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver Oxide
Silver oxide is the chemical compound with the formula Ag2O. It is a fine black or dark brown powder that is used to prepare other silver compounds. Preparation Silver oxide can be prepared by combining aqueous solutions of silver nitrate and an alkali hydroxide. This reaction does not afford appreciable amounts of silver hydroxide due to the favorable energetics for the following reaction: :2 AgOH -> Ag2O + H2O ( p''K'' = 2.875) With suitably controlled conditions, this reaction can be used to prepare Ag2O powder with properties suitable for several uses including as a fine grained conductive paste filler. Structure and properties Ag2O features linear, two-coordinate Ag centers linked by tetrahedral oxides. It is isostructural with Cu2O. It "dissolves" in solvents that degrade it. It is slightly soluble in water due to the formation of the ion and possibly related hydrolysis products. It is soluble in ammonia solution, producing active compound of Tollens' reagent. A sl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




.png)