|
DPEN
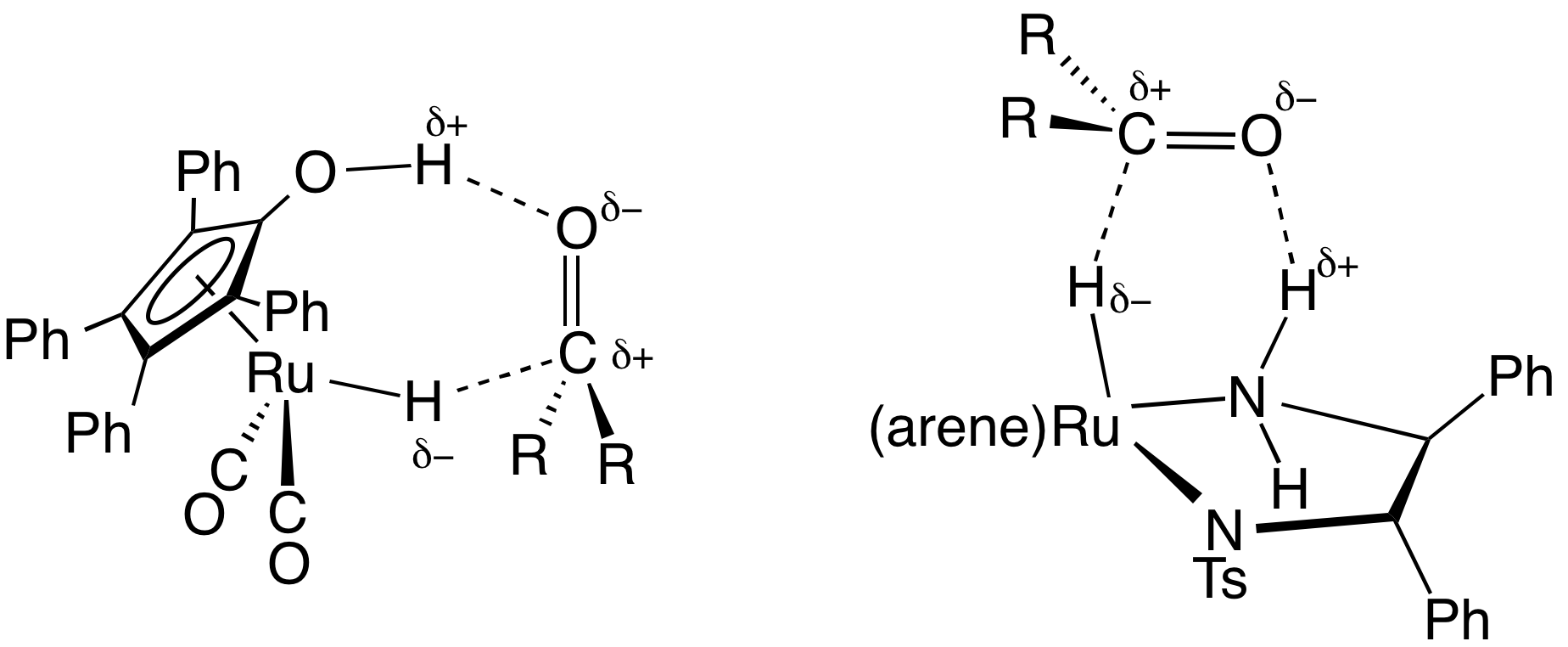
1,2-Diphenyl-1,2-ethylenediamine, DPEN, is an organic compound with the formula H2NCHPhCHPhNH2, where Ph is phenyl (C6H5). DPEN exists as three stereoisomers: meso and two enantiomers S,S- and R,R-. The chiral diastereomers are used in asymmetric hydrogenation. Both diastereomers are bidentate ligands. Preparation and optical resolution 1,2-Diphenyl-1,2-ethylenediamine can be prepared from benzil by reductive amination. DPEN can be obtained as both the chiral and meso diastereomers, depending on the relative stereochemistry of the two CHPhNH2 subunits. The chiral diastereomer, which is of greater value, can be resolved into the R,R- and S,S- enantiomers using tartaric acid as the resolving agent. In methanol, the R,R enantiomer has a specific rotation of ±sub>23 +106±1°. Asymmetric catalysis N- tosylated derivative, TsDPEN, is a ligand precursor for catalysts for asymmetric transfer hydrogenation. For example, (cymene)Ru(''S'',''S''-TsDPEN) catalyzes the hydrogenation of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ruthenium
Ruthenium is a chemical element with the Symbol (chemistry), symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most other chemicals. Russian-born scientist of Baltic-German ancestry Karl Ernst Claus discovered the element in 1844 at Kazan State University and named ruthenium in honor of Russian Empire, Russia. Ruthenium is usually found as a minor component of platinum ores; the annual production has risen from about 19 tonnes in 2009Summary. Ruthenium platinum.matthey.com, p. 9 (2009) to some 35.5 tonnes in 2017. Most ruthenium produced is used in wear-resistant electrical contacts and thick-film resistors. A minor application for ruthenium is in platinu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Asymmetric Hydrogenation
Asymmetric hydrogenation is a chemical reaction that adds two atoms of Hydrogen atom, hydrogen to a target (substrate) molecule with three-dimensional Enantioselective synthesis, spatial selectivity. Critically, this selectivity does not come from the target molecule itself, but from other reagents or catalysts present in the reaction. This allows spatial information (what chemists refer to as chirality) to transfer from one molecule to the target, forming the product as a single enantiomer. The chiral information is most commonly contained in a catalyst and, in this case, the information in a single molecule of catalyst may be transferred to many substrate molecules, amplifying the amount of chiral information present. Similar processes occur in nature, where a chiral molecule like an enzyme can catalyse the introduction of a chiral centre to give a product as a single enantiomer, such as amino acids, that a cell needs to function. By imitating this process, chemists can generate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzil
Benzil (i.e. Bz2, systematically known as 1,2-diphenylethane-1,2-dione) is the organic compound with the formula ( C6H5 CO)2, generally abbreviated ( PhCO)2. This yellow solid is one of the most common diketones. Its main use is as a photoinitiator in polymer chemistry.Hardo Siegel, Manfred Eggersdorfer "Ketones" in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH, 2002 by Wiley-VCH, Weinheim. Structure The compound's most noteworthy structural feature is the long carbon-carbon bond of 1.54 à , which indicates the absence of pi-bonding between the two carbonyl centers. The PhCO centers are planar, but the pair of benzoyl groups are twisted with respect to the other with a dihedral angle of 117°. In less hindered analogues (glyoxal, biacetyl, oxalic acid derivatives), the (RCO)2 group adopts a planar, anti-conformation. Applications Most benzil can be used as a photoinitiator in the free-radical curing of polymer networks. It absorbs ultraviolet radiation at a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamines
A diamine is an amine with exactly two amino groups. Diamines are used as monomers to prepare polyamides, polyimides, and polyureas. The term ''diamine'' refers mostly to primary diamines, as those are the most reactive. In terms of quantities produced, 1,6-diaminohexane (a precursor to Nylon 6-6) is most important, followed by ethylenediamine. Vicinal diamines (1,2-diamines) are a structural motif in many biological compounds and are used as ligands in coordination chemistry. Aliphatic diamines Linear * 1 carbon: methylenediamine (diaminomethane) of theoretical interest only * 2 carbons: ethylenediamine (1,2-diaminoethane). Related derivatives include the N-alkylated compounds, 1,1-dimethylethylenediamine, 1,2-dimethylethylenediamine, ethambutol, tetrakis(dimethylamino)ethylene, TMEDA. File:Ethylene_diamine.png, Ethylenediamine * 3 carbons: 1,3-diaminopropane (propane-1,3-diamine) * 4 carbons: putrescine (butane-1,4-diamine) * 5 carbons: cadaverine (pentane-1,5-diamine) F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nobel Prize In Chemistry
) , image = Nobel Prize.png , alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFRâą" then "NOBEL", and on the right, the text (smaller) "NATâą" then "MDCCCXXXIII" above, followed by (smaller) "OBâą" then "MDCCCXCVI" below. , awarded_for = Outstanding contributions in chemistry , presenter = Royal Swedish Academy of Sciences , location = Stockholm, Sweden , reward = 9 million SEK (2017) , year = 1901 , holder = Carolyn R. Bertozzi, Morten P. Meldal and Karl Barry Sharpless (2022) , most_awards = Frederick Sanger and Karl Barry Sharpless (2) , website nobelprize.org, previous = 2021 , year2=2022, main=2022, next=2023 The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Desymmetrization
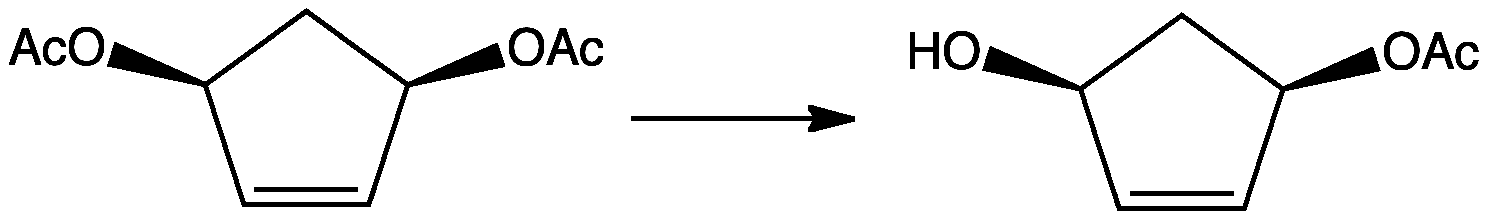
Desymmetrization in stereochemistry is the modification of a molecule that results in the loss of one or more symmetry elements. A common application of this class of reactions involves the introduction of chirality.Willis, Michael C. "Enantioselective desymmetrization" Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry 1999, pp. 1765-1784. Formally, such conversions required the loss of an improper axis of rotation (mirror plane, center of inversion, rotation-reflection axis). In other words, desymmetrisations convert prochiral precursors into chiral products. Examples Typical substrates are epoxides, diols, dienes, and carboxylic acid anhydrides. One example is the conversion of cis-3,5-diacetoxycyclopentene to monoacetate. In this transformation, the plane of symmetry in the precursor is lost, and the product is asymmetric. The desymmetrisation itself is not usually considered useful. The ''enantioselective'' desymmetrisation how ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RyĆji Noyori
is a Japanese chemist. He won the Nobel Prize in Chemistry in 2001, Noyori shared a half of the prize with William S. Knowles for the study of chirally catalyzed hydrogenations; the second half of the prize went to K. Barry Sharpless for his study in chirally catalyzed oxidation reactions (Sharpless epoxidation). Education and career RyĆji Noyori was born in Kobe, Japan. Early in his school days Ryoji was interested in physics. His interest was kindled by the famous physicist Hideki Yukawa (1949 Nobel Prize in Physics winner), a close friend of his father. Later, he became fascinated with chemistry, after hearing a presentation on nylon at an industrial exposition. He saw the power of chemistry as being the ability to "produce high value from almost nothing". He was a student at the School of Engineering (Department of Industrial Chemistry) of the Kyoto University, where he graduated in 1961. He subsequently obtained a Master's degree in Industrial Chemistry from the Gradua ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formate
Formate (IUPAC name: methanoate) is the conjugate base of formic acid. Formate is an anion () or its derivatives such as ester of formic acid. The salts and esters are generally colorless.Werner Reutemann and Heinz Kieczka "Formic Acid" in ''Ullmann's Encyclopedia of Industrial Chemistry'' 2002, Wiley-VCH, Weinheim. Fundamentals When dissolved in water, formic acid converts to formate: : Formate is a planar anion. The two oxygen atoms are equivalent and bear a partial negative charge. The remaining C-H bond is not acidic. Biochemistry : Formate is a common C-1 source in living systems. It is formed from many precursors including choline, serine, and sarcosine. It provides a C-1 source in the biosynthesis of some nucleic acids. Formate (or formic acid) is ina leaving group in the demethylation of some sterols.. These conversions are catalyzed by aromatase enzymes using O2 as the oxidant. Specific conversions include testosterone to estradiol and androstenedione to estrone ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzoin (organic Compound)
Benzoin ( or ) is an organic compound with the formula PhCH(OH)C(O)Ph. It is a hydroxy ketone attached to two phenyl groups. It appears as off-white crystals, with a light camphor-like odor. Benzoin is synthesized from benzaldehyde in the benzoin condensation. It is chiral and it exists as a pair of enantiomers: (''R'')-benzoin and (''S'')-benzoin. Benzoin is ''not'' a constituent of benzoin resin obtained from the Styrax, benzoin tree ''(Styrax)'' or tincture of benzoin. The main component in these natural products is benzoic acid. History Benzoin was first reported in 1832 by Justus von Liebig and Friedrich Woehler during their research on oil of bitter almond, which is benzaldehyde with traces of hydrocyanic acid. The catalytic synthesis by the benzoin condensation was improved by Nikolay Zinin during his time with Liebig. Uses The main uses of benzoin are as a precursor to benzil, which is a photoinitiator.Hardo Siegel, Manfred Eggersdorfer "Ketones" in Ullmann's Encyclope ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transfer Hydrogenation
In chemistry, transfer hydrogenation is a chemical reaction involving the addition of hydrogen to a compound from a source other than molecular . It is applied in laboratory and industrial organic synthesis to saturate organic compounds and reduce ketones to alcohols, and imines to amines. It avoids the need for high-pressure molecular used in conventional hydrogenation. Transfer hydrogenation usually occurs at mild temperature and pressure conditions using organic or organometallic catalysts, many of which are chiral, allowing efficient asymmetric synthesis. It uses hydrogen donor compounds such as formic acid, isopropanol or dihydroanthracene, dehydrogenating them to , acetone, or anthracene respectively. Often, the donor molecules also function as solvents for the reaction. A large scale application of transfer hydrogenation is coal liquefaction using "donor solvents" such as tetralin. Organometallic catalysts In the area of organic synthesis, a useful family of hydrogen-trans ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a Catalysis, catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated and unsaturated compounds, saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces Double bond, double and Triple bond, triple bonds in hydrocarbons. Process Hydrogenation has three components, the Saturated and unsaturated compounds, unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The redox, reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst. Related or competing reactions The same ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |