|
Dubnium
Dubnium is a synthetic chemical element with the symbol Db and atomic number 105. It is highly radioactive: the most stable known isotope, dubnium-268, has a half-life of about 16 hours. This greatly limits extended research on the element. Dubnium does not occur naturally on Earth and is produced artificially. The Soviet Joint Institute for Nuclear Research (JINR) claimed the first discovery of the element in 1968, followed by the American Lawrence Berkeley Laboratory in 1970. Both teams proposed their names for the new element and used them without formal approval. The long-standing dispute was resolved in 1993 by an official investigation of the discovery claims by the Transfermium Working Group, formed by the International Union of Pure and Applied Chemistry and the International Union of Pure and Applied Physics, resulting in credit for the discovery being officially shared between both teams. The element was formally named ''dubnium'' in 1997 after the town of Dubna, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Group 5 Element
Group 5 is a group of elements in the periodic table. Group 5 contains vanadium (V), niobium (Nb), tantalum (Ta) and dubnium (Db). This group lies in the d-block of the periodic table. This group is sometimes called the vanadium group or vanadium family after its lightest member; however, the group itself has not acquired a trivial name because it belongs to the broader grouping of the transition metals. "Group 5" is the new IUPAC name for this group; the old style name was "''group VB''" in the old US system (CAS) or "''group VA''" in the European system (old IUPAC). Group 5 must not be confused with the group with the old-style group crossed names of either ''VA'' (US system, CAS) or ''VB'' (European system, old IUPAC). ''That'' group is now called the pnictogens or group 15. As is typical for early transition metals, niobium and tantalum have only the group oxidation state of +5 as a major one, and are quite electropositive and have a less rich coordination chemistry. Due to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
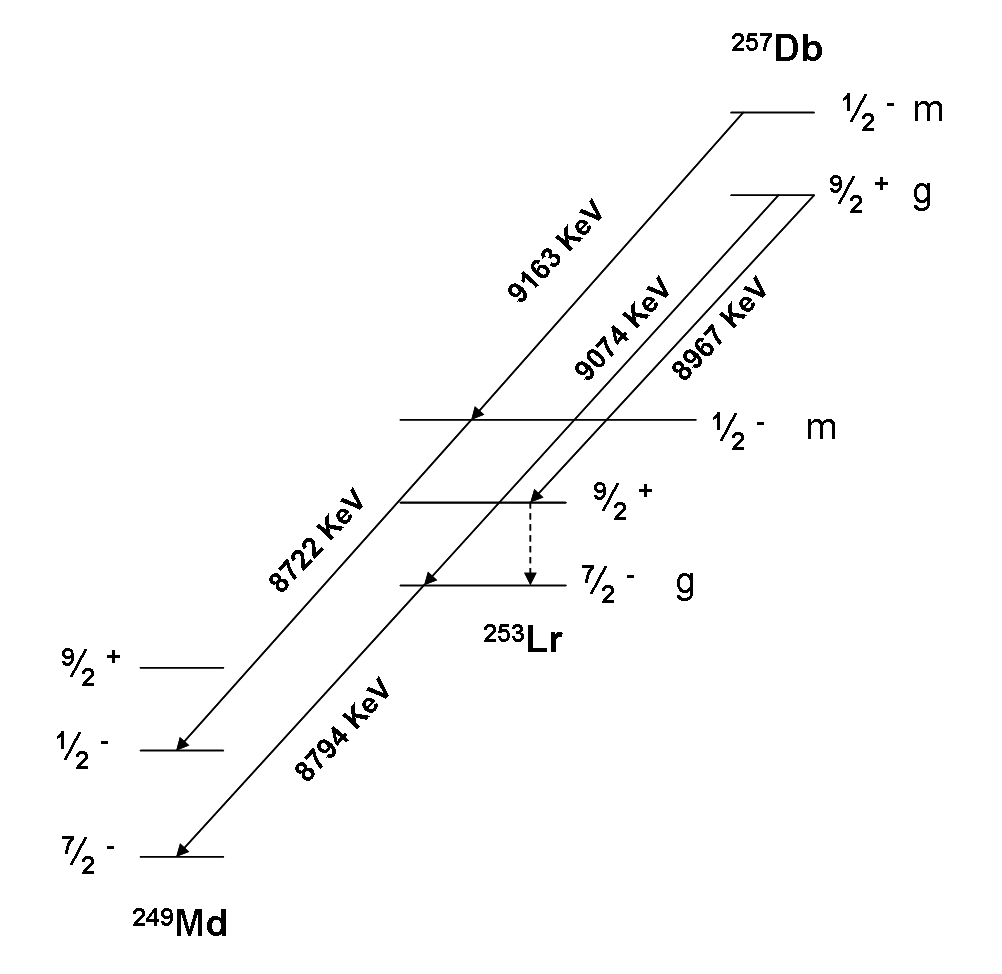
Isotopes Of Dubnium
Dubnium (105Db) is a synthetic element, thus a standard atomic weight cannot be given. Like all synthetic elements, it has no stable isotopes. The first isotope to be synthesized was 261Db in 1968. The 13 known radioisotopes are from 255Db to 270Db, and 1–3 isomers. The longest-lived known isotope is 268Db with a half-life of 16 hours. List of isotopes , - , rowspan=2, 255Db , rowspan=2 style="text-align:right" , 105 , rowspan=2 style="text-align:right" , 150 , rowspan=2, 255.10707(45)# , rowspan=2, , α (~50%) , 251Lr , rowspan=2, , - , SF (~50%) , (various) , - , rowspan=3, 256Db , rowspan=3 style="text-align:right" , 105 , rowspan=3 style="text-align:right" , 151 , rowspan=3, 256.10789(26)# , rowspan=3, 1.9(4) s[] , α (~64%) , 252Lr , rowspan=3, , - , SF (~0.02%) , (various) , - , beta decay, β+ (~36%) , 256Rf , - , rowspan=3, 257Db , rowspan=3 style="text-align:right" , 105 , rowspan=3 style="text-align:right" , 152 , rowspan=3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transfermium Wars
The names for the chemical elements 104 to 106 were the subject of a major controversy starting in the 1960s, described by some nuclear chemists as the Transfermium Wars because it concerned the elements following fermium (element 100) on the periodic table. This controversy arose from disputes between American scientists and Soviet scientists as to which had first isolated these elements. The final resolution of this controversy in 1997 also decided the names of elements 107 to 109. Controversy By convention, naming rights for newly discovered chemical elements go to their discoverers. For elements 104, 105, and 106, there was a controversy between Soviet researchers at the Joint Institute for Nuclear Research and American researchers at Lawrence Berkeley National Laboratory regarding which group had discovered them first. Both parties suggested their own names for elements 104 and 105, not recognizing the other's name. The American name of seaborgium for element 106 was also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Symbol (chemistry)
Chemical symbols are the abbreviations used in chemistry for chemical elements, functional groups and chemical compounds. Element symbols for chemical elements normally consist of one or two letters from the Latin alphabet and are written with the first letter capitalised. History Earlier symbols for chemical elements stem from classical Latin and Greek vocabulary. For some elements, this is because the material was known in ancient times, while for others, the name is a more recent invention. For example, Pb is the symbol for lead (''plumbum'' in Latin); Hg is the symbol for mercury (''hydrargyrum'' in Greek); and He is the symbol for helium (a new Latin name) because helium was not known in ancient Roman times. Some symbols come from other sources, like W for tungsten (''Wolfram'' in German) which was not known in Roman times. A three-letter temporary symbol may be assigned to a newly synthesized (or not yet synthesized) element. For example, "Uno" was the temporary symbol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthetic Element
A synthetic element is one of 24 known chemical elements that do not occur naturally on Earth: they have been created by human manipulation of fundamental particles in a nuclear reactor, a particle accelerator, or the explosion of an atomic bomb; thus, they are called "synthetic", "artificial", or "man-made". The synthetic elements are those with atomic numbers 95–118, as shown in purple on the accompanying periodic table: these 24 elements were first created between 1944 and 2010. The mechanism for the creation of a synthetic element is to force additional protons into the nucleus of an element with an atomic number lower than 95. All synthetic elements are unstable, but they decay at widely varying rates: the half-lives of their longest-lived isotopes range from microseconds to millions of years. Five more elements that were created artificially are strictly speaking not ''synthetic'' because they were later found in nature in trace quantities: 43Tc, 61Pm, 85At, 93Np, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Niobium
Niobium is a chemical element with chemical symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and ductile transition metal. Pure niobium has a Mohs hardness rating similar to pure titanium, and it has similar ductility to iron. Niobium oxidizes in Earth's atmosphere very slowly, hence its application in jewelry as a hypoallergenic alternative to nickel. Niobium is often found in the minerals pyrochlore and columbite, hence the former name "columbium". Its name comes from Greek mythology: Niobe, daughter of Tantalus, the namesake of tantalum. The name reflects the great similarity between the two elements in their physical and chemical properties, which makes them difficult to distinguish. English chemist Charles Hatchett reported a new element similar to tantalum in 1801 and named it columbium. In 1809, English chemist William Hyde Wollaston wrongly concluded that tantalum and columbium were identical. German chemist Heinrich ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lawrence Berkeley Laboratory
Lawrence Berkeley National Laboratory (LBNL), commonly referred to as the Berkeley Lab, is a United States national laboratory that is owned by, and conducts scientific research on behalf of, the United States Department of Energy. Located in the hills of Berkeley, California, the lab overlooks the campus of the University of California, Berkeley, and is managed by the University of California system. History 1931–1941 The laboratory was founded on August 26, 1931, by Ernest Lawrence, as the Radiation Laboratory of the University of California, Berkeley, associated with the Physics Department. It centered physics research around his new instrument, the cyclotron, a type of particle accelerator for which he was awarded the Nobel Prize in Physics in 1939. Throughout the 1930s, Lawrence pushed to create larger and larger machines for physics research, courting private philanthropists for funding. He was the first to develop a large team to build big projects to make discoveries ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transactinide Chemistry Apparatus Dubna
Superheavy elements, also known as transactinide elements, transactinides, or super-heavy elements, are the chemical elements with atomic number greater than 103. The superheavy elements are those beyond the actinides in the periodic table; the last actinide is lawrencium (atomic number 103). By definition, superheavy elements are also transuranium elements, i.e., having atomic numbers greater than that of uranium (92). Depending on the definition of group 3 adopted by authors, lawrencium may also be included to complete the 6d series. Glenn T. Seaborg first proposed the actinide concept, which led to the acceptance of the actinide series. He also proposed a transactinide series ranging from element 104 to 121 and a superactinide series approximately spanning elements 122 to 153 (although more recent work suggests the end of the superactinide series to occur at element 157 instead). The transactinide seaborgium was named in his honor. Superheavy elements are radioactive and have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dubna
Dubna ( rus, Дубна́, p=dʊbˈna) is a town in Moscow Oblast, Russia. It has a status of '' naukograd'' (i.e. town of science), being home to the Joint Institute for Nuclear Research, an international nuclear physics research center and one of the largest scientific foundations in the country. It is also home to MKB Raduga, a defense aerospace company specializing in design and production of missile systems, as well as to the Russia's largest satellite communications center owned by Russian Satellite Communications Company. The modern town was developed in the middle of the 20th century and town status was granted to it in 1956. Population: Geography The town is above sea level, situated approximately north of Moscow, on the Volga River, just downstream from the Ivankovo Reservoir. The reservoir is formed by a hydroelectric dam across the Volga situated within the town borders. The town lies on both banks of the Volga. The western boundary of the town is defined by t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nobelium
Nobelium is a synthetic chemical element with the symbol No and atomic number 102. It is named in honor of Alfred Nobel, the inventor of dynamite and benefactor of science. A radioactive metal, it is the tenth transuranic element and is the penultimate member of the actinide series. Like all elements with atomic number over 100, nobelium can only be produced in particle accelerators by bombarding lighter elements with charged particles. A total of twelve nobelium isotopes are known to exist; the most stable is 259No with a half-life of 58 minutes, but the shorter-lived 255No (half-life 3.1 minutes) is most commonly used in chemistry because it can be produced on a larger scale. Chemistry experiments have confirmed that nobelium behaves as a heavier homolog to ytterbium in the periodic table. The chemical properties of nobelium are not completely known: they are mostly only known in aqueous solution. Before nobelium's discovery, it was predicted that it would show a stable ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Discovery Of The Chemical Elements
The discovery of the 118 chemical elements known to exist as of 2022 is presented in chronological order. The elements are listed generally in the order in which each was first defined as the pure element, as the exact date of discovery of most elements cannot be accurately determined. There are plans to synthesize more elements, and it is not known how many elements are possible. Each element's name, atomic number, year of first report, name of the discoverer, and notes related to the discovery are listed. Periodic table of elements Ancient discoveries Modern discoveries Graphics See also * History of the periodic table * Periodic table * Extended periodic table * ''The Mystery of Matter: Search for the Elements'' (2014/2015 PBS film) * Transfermium Wars References External linksHistory of the Origin of the Chemical Elements and Their DiscoverersLast updated by Boris Pritychenko on March 30, 2004 [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Moscow Oblast
Moscow Oblast ( rus, Моско́вская о́бласть, r=Moskovskaya oblast', p=mɐˈskofskəjə ˈobləsʲtʲ), or Podmoskovye ( rus, Подмоско́вье, p=pədmɐˈskovʲjə, literally " under Moscow"), is a federal subject of Russia (an oblast). With a population of 7,095,120 ( 2010 Census) living in an area of , it is one of the most densely populated regions in the country and is the second most populous federal subject. The oblast has no official administrative center; its public authorities are located in Moscow and Krasnogorsk (Moscow Oblast Duma and government), and also across other locations in the oblast.According to Article 24 of the Charter of Moscow Oblast, the government bodies of the oblast are located in the city of Moscow and throughout the territory of Moscow Oblast. However, Moscow is not named the official administrative center of the oblast. Located in European Russia between latitudes 54° and 57° N and longitudes 35° and 41° ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.jpg)


