|
Dppp
1,3-Bis(diphenylphosphino)propane (dppp) is an organophosphorus compound with the formula PhP(CH)PPh. The compound is a white solid that is soluble in organic solvents. It is slightly air-sensitive, degrading in air to the phosphine oxide. It is classified as a diphosphine ligand in coordination chemistry and homogeneous catalysis. The diphosphine can be prepared by the reaction of lithium diphenylphosphide and 1,3-dichloropropane (Ph = CH): : 2 PhPLi + CHCl → PhP(CH)PPh + 2 LiCl However, it can be synthesised via a much more controllable (and cheaper) route, via metal-halogen exchange and then metathesis: :Br(CH)Br + 2 BuLi → Li(CH)Li + 2 BuBr :Li(CH)Li + 2 PCl → ClP(CH)PCl + 2 LiCl :ClP(CH)PCl + 4 PhLi → PhP(CH)PPh + 4 LiCl Coordination chemistry and use as co-catalyst The diphosphine serves as a bidentate ligand forming six-membered CPM chelate ring with a natural bite angle of 91°. For example, the complex dichloro(1,3-bis(diphenylphosphino)propane)nickel i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diphosphines
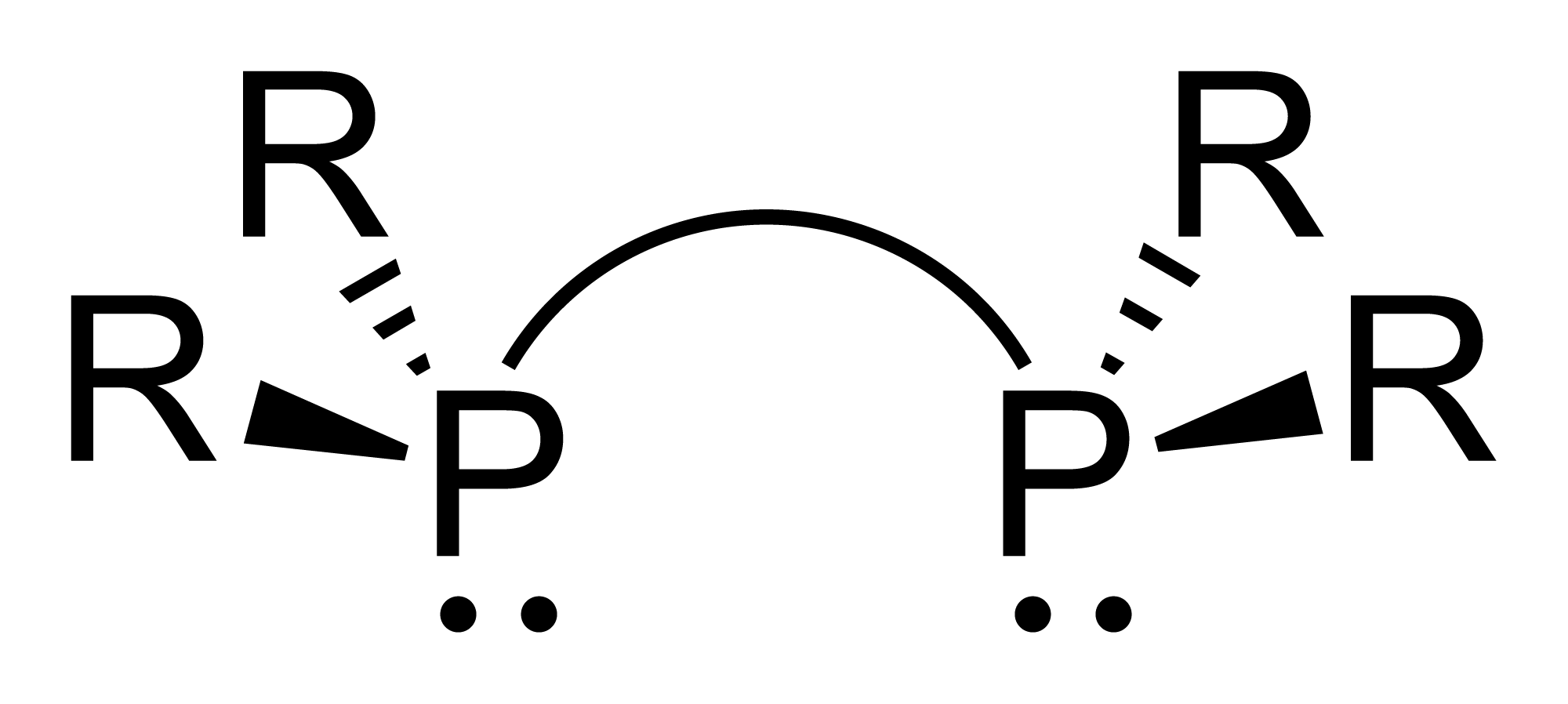
Diphosphines, sometimes called bisphosphanes, are organophosphorus compounds most commonly used as bidentate phosphine ligands in inorganic and organometallic chemistry. They are identified by the presence of two phosphino groups linked by a backbone, and are usually chelating. A wide variety of diphosphines have been synthesized with different linkers and R-groups. Alteration of the linker and R-groups alters the electronic and steric properties of the ligands which can result in different coordination geometries and catalytic behavior in homogeneous catalysts. Synthesis 222px, Chlorodiisopropylphosphine is a popular building block for the preparation of diphosphines. From phosphide building blocks Many widely used diphosphine ligands have the general formula Ar2P(CH2)nPAr2. These compounds can be prepared from the reaction of X(CH2)nX (X=halogen) and MPPh2 (M = alkali metal): :Cl(CH2)nCl + 2 NaPPh2 → Ph2P(CH2)nPPh2 + 2 NaCl Diphosphine ligands can also be prepare ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diphosphines
Diphosphines, sometimes called bisphosphanes, are organophosphorus compounds most commonly used as bidentate phosphine ligands in inorganic and organometallic chemistry. They are identified by the presence of two phosphino groups linked by a backbone, and are usually chelating. A wide variety of diphosphines have been synthesized with different linkers and R-groups. Alteration of the linker and R-groups alters the electronic and steric properties of the ligands which can result in different coordination geometries and catalytic behavior in homogeneous catalysts. Synthesis 222px, Chlorodiisopropylphosphine is a popular building block for the preparation of diphosphines. From phosphide building blocks Many widely used diphosphine ligands have the general formula Ar2P(CH2)nPAr2. These compounds can be prepared from the reaction of X(CH2)nX (X=halogen) and MPPh2 (M = alkali metal): :Cl(CH2)nCl + 2 NaPPh2 → Ph2P(CH2)nPPh2 + 2 NaCl Diphosphine ligands can also be prepare ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel(II) Chloride
Nickel(II) chloride (or just nickel chloride) is the chemical compound NiCl2. The anhydrous salt is yellow, but the more familiar hydrate NiCl2·6H2O is green. Nickel(II) chloride, in various forms, is the most important source of nickel for chemical synthesis. The nickel chlorides are deliquescent, absorbing moisture from the air to form a solution. Nickel salts have been shown to be carcinogenic to the lungs and nasal passages in cases of long-term inhalation exposure. Production and syntheses The largest scale production of nickel chloride involves the extraction with hydrochloric acid of nickel matte and residues obtained from roasting refining nickel-containing ores. Nickel chloride is not usually prepared in the laboratory because it is inexpensive and has a long shelf-life. Heating the hexahydrate in the range 66–133.°C gives the yellowish dihydrate, NiCl2·2H2O. The hydrates convert to the anhydrous form upon heating in thionyl chloride or by heating under a stream ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heck Reaction
The Heck reaction (also called the Mizoroki–Heck reaction) is the chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence of a base and a palladium catalyst (or palladium nanomaterial-based catalyst) to form a substituted alkene. It is named after Tsutomu Mizoroki and Richard F. Heck. Heck was awarded the 2010 Nobel Prize in Chemistry, which he shared with Ei-ichi Negishi and Akira Suzuki, for the discovery and development of this reaction. This reaction was the first example of a carbon-carbon bond-forming reaction that followed a Pd(0)/Pd(II) catalytic cycle, the same catalytic cycle that is seen in other Pd(0)-catalyzed cross-coupling reactions. The Heck reaction is a way to substitute alkenes. History The original reaction by Tsutomu Mizoroki (1971) describes the coupling between iodobenzene and styrene in methanol to form stilbene at 120 °C (autoclave) with potassium acetate base and palladium chloride catalysis. This work was an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyketone
Polyketones are a family of high-performance thermoplastic polymers. The polar ketone groups in the polymer backbone of these materials gives rise to a strong attraction between polymer chains, which increases the material's melting point (255 °C for copolymer (carbon monoxide ethylene), 220 °C for terpolymer (carbon monoxide, ethylene, propylene). Trade names include Poketone, Carilon, Karilon, Akrotek, and Schulaketon. Such materials also tend to resist solvents and have good mechanical properties. Unlike many other engineering plastics, aliphatic polyketones such as Shell Chemicals' Carilon are relatively easy to synthesize and can be derived from inexpensive monomers. Carilon is made with a palladium(II) catalyst from ethylene and carbon monoxide. A small fraction of the ethylene is generally replaced with propylene to reduce the melting point somewhat. Shell Chemical commercially launched Carilon thermoplastic polymer in the U.S. in 1996, but discontinued it in 200 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds). Ethylene is widely used in the chemical industry, and its worldwide production (over 150 million tonnes in 2016) exceeds that of any other organic compound. Much of this production goes toward polyethylene, a widely used plastic containing polymer chains of ethylene units in various chain lengths. Ethylene is also an important natural plant hormone and is used in agriculture to force the ripening of fruits. The hydrate of ethylene is ethanol. Structure and properties This hydrocarbon has four hydrogen atoms bound to a pair of carbon atoms that are connected by a double bond. All six atoms that comprise ethylene are coplanar. The H-C-H angle is 117.4°, close to the 120° for ideal sp² hybridized carbon. The molecule is also relatively weak: rota ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest molecule of the oxocarbon family. In coordination complexes the carbon monoxide ligand is called carbonyl. It is a key ingredient in many processes in industrial chemistry. The most common source of carbon monoxide is the partial combustion of carbon-containing compounds, when insufficient oxygen or heat is present to produce carbon dioxide. There are also numerous environmental and biological sources that generate and emit a significant amount of carbon monoxide. It is important in the production of many compounds, including drugs, fragrances, and fuels. Upon emission into the atmosphere, carbon monoxide affects several processes that contribute to climate change. Carbon monoxide has important biological roles across phylogenetic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired by her when she slew Pallas. Palladium, platinum, rhodium, ruthenium, iridium and osmium form a group of elements referred to as the platinum group metals (PGMs). They have similar chemical properties, but palladium has the lowest melting point and is the least dense of them. More than half the supply of palladium and its congener platinum is used in catalytic converters, which convert as much as 90% of the harmful gases in automobile exhaust (hydrocarbons, carbon monoxide, and nitrogen dioxide) into nontoxic substances (nitrogen, carbon dioxide and water vapor). Palladium is also used in electronics, dentistry, medicine, hydrogen purification, chemical applications, groundwate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kumada Coupling
In organic chemistry, the Kumada coupling is a type of cross coupling reaction, useful for generating carbon–carbon bonds by the reaction of a Grignard reagent and an organic halide. The procedure uses transition metal catalysts, typically nickel or palladium, to couple a combination of two alkyl, aryl or vinyl groups. The groups of Robert Corriu and Makoto Kumada reported the reaction independently in 1972. The reaction is notable for being among the first reported catalytic cross-coupling methods. Despite the subsequent development of alternative reactions (Suzuki reaction, Suzuki, Sonogashira coupling, Sonogashira, Stille coupling, Stille, Hiyama coupling, Hiyama, Negishi coupling, Negishi), the Kumada coupling continues to be employed in many Chemical synthesis, synthetic applications, including the industrial-scale production of aliskiren, a hypertension medication, and polythiophenes, useful in organic electronic devices. History The first investigations into the cat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphorus Compound
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents. Organophosphorus chemistry is the corresponding science of the properties and reactivity of organophosphorus compounds. Phosphorus, like nitrogen, is in group 15 of the periodic table, and thus phosphorus compounds and nitrogen compounds have many similar properties. The definition of organophosphorus compounds is variable, which can lead to confusion. In industrial and environmental chemistry, an organophosphorus compound need contain only an organic substituent, but need not have a direct phosphorus-carbon (P-C) bond. Thus a large proportion of pesticides (e.g., malathion), are often included in this class of compounds. Phosphorus can adopt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Air-sensitive
Air sensitivity is a term used, particularly in chemistry, to denote the reactivity of chemical compounds with some constituent of air. Most often, reactions occur with atmospheric oxygen (O2) or water vapor (H2O), although reactions with the other constituents of air such as carbon monoxide (CO), carbon dioxide (CO2), and nitrogen (N2) are also possible. Method A variety of air-free techniques have been developed to handle air-sensitive compounds. Two main types of equipment are gloveboxes and Schlenk lines. Glove boxes are sealed cabinets filled with an inert gas such as argon or nitrogen. Normal laboratory equipment can be set up in the glovebox, and manipulated by the use of gloves that penetrate its walls. The atmosphere can be regulated to approximately atmospheric pressure and set to be pure nitrogen or other gas with which the chemicals will not react. Chemicals and equipment can be transferred in and out via an airlock. A Schlenk line is a vacuum and inert-gas dual-ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bite Angle
In coordination chemistry the bite angle is the ligand–metal–ligand bond angle of coordination complex containing a bidentate ligand. This geometric parameter is used to classify chelation, chelating ligands, including those in organometallic complexes. It is most often discussed in terms of catalysis, as changes in bite angle can affect not just the activity and selectivity of a catalytic reaction but even allow alternative reaction pathways to become accessible. Although the parameter can be applied generally to any chelating ligand, it is commonly applied to describe diphosphine ligands, as they can adopt a wide range of bite angles. Diamines Diamines form a wide range of coordination complexes. They typically form 5- and 6-membered chelate rings. Examples of the former include ethylenediamine and 2,2'-bipyridine, 2,2′-bipyridine. Six-membered chelate rings are formed by 1,3-diaminopropane. The bite angle in such complexes is usually near 90°. Longer chain diamines, whi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

_complexes_in_aqueous_solution.jpg)


2.jpg)


-3D-balls.png)