|
Dobu
2,5-Dimethoxy-4-butylamphetamine (DOBU) is a lesser-known psychedelic drug and a substituted Amphetamine. DOBU was first synthesized by Alexander Shulgin. In his book '' PiHKAL (Phenethylamines i Have Known And Loved)'', only low dosages of 2–3 mg were tested, with the duration simply listed as "very long". DOBU produces paresthesia and difficulty sleeping, but with few other effects. Compared to shorter chain homologues such as DOM, DOET and DOPR which are all potent hallucinogens, DOBU has an even stronger 5-HT2 binding affinity but fails to substitute for hallucinogens in animals or produce hallucinogenic effects in humans, suggesting it has low efficacy and is thus an antagonist or weak partial agonist In pharmacology, partial agonists are drugs that bind to and activate a given receptor, but have only partial efficacy at the receptor relative to a full agonist. They may also be considered ligands which display both agonistic and antagonistic e ... at the 5-HT2A recep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Psychedelics, Dissociatives And Deliriants
Hallucinogens are a large, diverse class of psychoactive drugs that can produce altered states of consciousness characterized by major alterations in thought, mood, and perception as well as other changes. Most hallucinogens can be categorized as either being psychedelics, dissociatives, or deliriants. However, certain hallucinogens such as Fly agaric as well as other gabaergic hallucinogenics are more often considered to technically be hypnotics, therefore indicating another separate subcategory of drugs which can substantially alter visual perception. Etymology The word ''hallucinogen'' is derived from the word ''hallucination''. The term ''hallucinate'' dates back to around 1595–1605, and is derived from the Latin ''hallūcinātus'', the past participle of ''(h)allūcināri'', meaning "to wander in the mind." Characteristics Leo Hollister gave five criteria for classifying a drug as hallucinogenic.Glennon RA. Classical drugs: an introductory overview. In Lin GC and Gle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituted Amphetamine
Substituted amphetamines are a class of compounds based upon the amphetamine structure; it includes all derivative compounds which are formed by replacing, or substituting, one or more hydrogen atoms in the amphetamine core structure with substituents. The compounds in this class span a variety of pharmacological subclasses, including stimulants, empathogens, and hallucinogens, among others. Examples of substituted amphetamines are amphetamine (itself), methamphetamine, ephedrine, cathinone, phentermine, mephentermine, bupropion, methoxyphenamine, selegiline, amfepramone (diethylpropion), pyrovalerone, MDMA (ecstasy), and DOM (STP). Some of amphetamine's substituted derivatives occur in nature, for example in the leaves of ''Ephedra'' and khat plants. Amphetamine was first produced at the end of the 19th century. By the 1930s, amphetamine and some of its derivative compounds found use as decongestants in the symptomatic treatment of colds and also occasionally as psychoac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alexander Shulgin
Alexander Theodore "Sasha" Shulgin (June 17, 1925 – June 2, 2014) was an American medicinal chemist, biochemist, organic chemist, pharmacologist, psychopharmacologist, and author. He is credited with introducing 3,4-methylenedioxymethamphetamine (MDMA, commonly known as "ecstasy") to psychologists in the late 1970s for psychopharmaceutical use and for the discovery, synthesis and personal bioassay of over 230 psychoactive compounds for their psychedelic and entactogenic potential. In 1991 and 1997, he and his wife Ann Shulgin compiled the books '' PiHKAL'' and ''TiHKAL'' (standing for ''Phenethylamines'' and ''Tryptamines I Have Known And Loved''), from notebooks that extensively described their work and personal experiences with these two classes of psychoactive drugs. Shulgin performed seminal work into the descriptive synthesis of many of these compounds. Some of Shulgin's noteworthy discoveries include compounds of the 2C* family (such as 2C-B) and compounds of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paresthesia
Paresthesia is an abnormal sensation of the skin (tingling, pricking, chilling, burning, numbness) with no apparent physical cause. Paresthesia may be transient or chronic, and may have any of dozens of possible underlying causes. Paresthesias are usually painless and can occur anywhere on the body, but most commonly occur in the arms and legs. The most familiar kind of paresthesia is the sensation known as "pins and needles" after obdormition, having a limb "fall asleep". A less well-known and uncommon paresthesia is formication, the sensation of insects crawling on the skin. Causes Transient Paresthesias of the hands, feet, legs, and arms are common transient symptoms. The briefest electric shock type of paresthesia can be caused by tweaking the ulnar nerve near the elbow; this phenomenon is colloquially known as bumping one's "funny bone". Similar brief shocks can be experienced when any other nerve is tweaked (e.g. a pinched neck nerve may cause a brief shock-like paresthesi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sleeping
Sleep is a sedentary state of mind and body. It is characterized by altered consciousness, relatively inhibited sensory activity, reduced muscle activity and reduced interactions with surroundings. It is distinguished from wakefulness by a decreased ability to react to stimuli, but more reactive than a coma or disorders of consciousness, with sleep displaying different, active brain patterns. Sleep occurs in repeating periods, in which the body alternates between two distinct modes: REM sleep and non-REM sleep. Although REM stands for "rapid eye movement", this mode of sleep has many other aspects, including virtual paralysis of the body. Dreams are a succession of images, ideas, emotions, and sensations that usually occur involuntarily in the mind during certain stages of sleep. During sleep, most of the body's systems are in an anabolic state, helping to restore the immune, nervous, skeletal, and muscular systems; these are vital processes that maintain mood, memory, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2,5-Dimethoxy-4-methylamphetamine
2,5-Dimethoxy-4-methylamphetamine (DOM; known as STP, standing for "Serenity, Tranquility and Peace") is a psychedelic and a substituted amphetamine. It was first synthesized by Alexander Shulgin, and later reported in his book '' PiHKAL: A Chemical Love Story''. DOM is classified as a Schedule I substance in the United States, and is similarly controlled in other parts of the world. Internationally, it is a Schedule I drug under the Convention on Psychotropic Substances. It is generally taken orally. History STP was first synthesized and tested in 1963 by Alexander Shulgin, who was investigating the effect of 4-position substitutions on psychedelic amphetamines. In mid-1967, tablets containing 20 mg (later 10 mg) of STP were widely distributed in the Haight-Ashbury District of San Francisco under the name of STP, having been manufactured by underground chemists Owsley Stanley and Tim Scully. This short-lived appearance of STP on the black market proved disastrous ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DOET
2,5-Dimethoxy-4-ethylamphetamine (DOET, DOE, Hecate) is a psychedelic drug of the phenethylamine and amphetamine chemical classes. It was first synthesized by Alexander Shulgin, and was described in his book '' PiHKAL'' (''Phenethylamines i Have Known And Loved''). Chemistry DOET is in a class of compounds commonly known as substituted amphetamines; its full chemical name is 4-ethyl-2,5-dimethoxy-alpha-methylbenzeneethanamine, or 1-(2,5-dimethoxy-4-ethylphenyl)propan-2-amine. It has an active stereocenter and (R)-DOET is the more active enantiomer. DOET is an extremely rare compound and reports of its effects and toxicology in humans are sparse. However, like the more common 2,5-dimethoxy-amphetamine analogues DOB, DOI and DOM, it is a potent and long-acting psychedelic. Removal of the alpha-methyl moiety yields the 2-carbon analogue, commonly known as 2C-E, another psychedelic compound first synthesized by Dr. Alexander Shulgin. Pharmacology Similarly to related drugs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DOPR
2,5-Dimethoxy-4-propylamphetamine (DOPR) is a psychedelic drug of the phenethylamine and amphetamine chemical classes. It was first synthesized by Alexander Shulgin, and was described in his book '' PiHKAL'' (''Phenethylamines i Have Known And Loved''). Shulgin described DOPR is a "heavy duty psychedelic", complete with alterations of the thought process and visual distortion. Very little data exists about the pharmacological properties, metabolism, and toxicity of DOPR. The alternative structural isomer DOIP, with a 4-isopropyl substitution, is also known but is around ten times weaker than DOPR, with an active dose of some 20–30 mg (as compared to 2–5 mg for DOPR). See also * DOPF * DOx 4-Substituted-2,5-dimethoxyamphetamines (DO''x'') is a chemical class of substituted amphetamine derivatives featuring methoxy groups at the 2- and 5- positions of the phenyl ring, and a substituent such as alkyl or halogen at the 4- po ... References ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dissociation Constant
In chemistry, biochemistry, and pharmacology, a dissociation constant (K_D) is a specific type of equilibrium constant that measures the propensity of a larger object to separate (dissociate) reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into its component ions. The dissociation constant is the inverse of the association constant. In the special case of salts, the dissociation constant can also be called an ionization constant. For a general reaction: : A_\mathit B_\mathit \mathit A + \mathit B in which a complex \ce_x \ce_y breaks down into ''x'' A subunits and ''y'' B subunits, the dissociation constant is defined as : K_D = \frac where and ''x'' B''y''are the equilibrium concentrations of A, B, and the complex A''x'' B''y'', respectively. One reason for the popularity of the dissociation constant in biochemistry and pharmacology is that in the frequently encount ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intrinsic Activity
Intrinsic activity (IA) and efficacy refer to the relative ability of a drug-receptor complex to produce a maximum functional response. This must be distinguished from the affinity, which is a measure of the ability of the drug to bind to its molecular target, and the EC50, which is a measure of the potency of the drug and which is proportional to both efficacy and affinity. This use of the word "efficacy" was introduced by Stephenson (1956) to describe the way in which agonists vary in the response they produce, even when they occupy the same number of receptors. High efficacy agonists can produce the maximal response of the receptor system while occupying a relatively low proportion of the receptors in that system. There is a distinction between efficacy and intrinsic activity. Mechanism of efficacy Agonists of lower efficacy are not as efficient at producing a response from the drug-bound receptor, by stabilizing the active form of the drug-bound receptor. Therefore, they ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
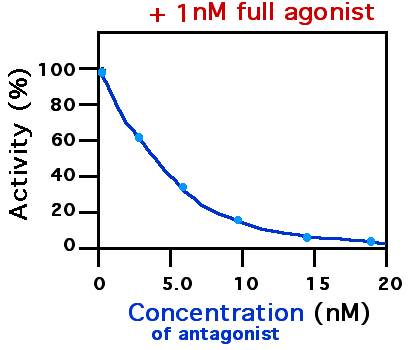
Antagonist (pharmacology)
A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of receptor proteins.Pharmacology Guide: In vitro pharmacology: concentration-response curves " '' GlaxoWellcome.'' Retrieved on December 6, 2007. They are sometimes called blockers; examples include alpha blockers, |