|
Diphosphene
Diphosphene is a compound having the formula (PH)2. It exists as two geometric isomers, ''E'' and ''Z''. Diphosphene is also the parent member of the entire class of diphosphene compounds with the formula (PR)2, where R is an organyl In organic and organometallic chemistry, an organyl group is an organic substituent with one (sometimes more) free valence(-s) at a carbon atom.. The term is often used in chemical patent literature to protect claims over a broad scope. Exam ... group. References {{Inorganic-compound-stub Phosphorus hydrides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diphosphenes
Diphosphene is a type of organophosphorus compound that has a phosphorus–phosphorus double bond, denoted by R-P=P-R'. These compounds are not common but are of theoretical interest. Normally, compounds with the empirical formula RP exist as rings. However, like other multiple bonds between heavy main-group elements, P=P double bonds can be stabilized by a large steric hindrance from the substitutions. The first isolated diphosphene bis(2,4,6-tri-tert-butylphenyl)diphosphene was exemplified by Masaaki Yoshifuji and his coworkers in 1981, in which diphosphene is stabilized by two bulky phenyl group. Synthesis Synthesis of aryl-substituted diphosphene In 1877, Köhler and Michaelis claimed that they synthesized the first isolated diphosphene (PhP=PPh). However, the molecular weight determination and X-ray crystallographic analysis later proved that this "diphosphene" only had a P-P single bond. Then the research to diphosphenes kept silent over almost 20 years until Masaaki ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine hydrate (). Hydrazine is mainly used as a foaming agent in preparing polymer foams, but applications also include its uses as a precursor to polymerization catalysts, pharmaceuticals, and agrochemicals, as well as a long-term storable propellant for in-space spacecraft propulsion. Additionally, hydrazine is used in various rocket fuels and to prepare the gas precursors used in air bags. Hydrazine is used within both nuclear and conventional electrical power plant steam cycles as an oxygen scavenger to control concentrations of dissolved oxygen in an effort to reduce corrosion. the world hydrazine hydrate market amounted to $350 million. About two million tons of hydrazine hydrate were used in foam blowing agents in 2015. Hydrazines r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triazane
Triazane is an inorganic compound with the chemical formula or . Triazane is the third simplest acyclic azane after ammonia and hydrazine. It can be synthesized from hydrazine but is unstable and cannot be isolated in the free base form, only as salt forms such as triazanium sulfate. Attempts to convert triazanium salts to the free base release only diazene and ammonia. Triazane was first synthesized as a ligand In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ... of the silver complex ion: tris(μ2-triazane-κ2''N''1,''N''3)disilver(2+). Triazane has also been synthesized in electron-irradiated ammonia ices and detected as a stable gas-phase product after sublimation.Forstel, Maksyutenko, Jones, Sun, Chen, Chang, & Kaiser. "Detection of the Elusive Triazane Molecule () in the Gas P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diazene
Diimide, also called diazene or diimine, is a compound having the formula (NH)2. It exists as two geometric isomers, ''E'' (''trans'') and ''Z'' (''cis''). The term diazene is more common for organic derivatives of diimide. Thus, azobenzene is an example of an organic diazene. Synthesis A traditional route to diimide involves oxidation of hydrazine with hydrogen peroxide or air. Alternatively the hydrolysis of diethyl azodicarboxylate or azodicarbonamide affords diimide: :(NCOOH)2 → (NH)2 + 2 CO2 Nowadays, diimide is generated by thermal decomposition of 2,4,6‐triisopropylbenzenesulfonylhydrazide. Because of its instability, diimide is generated and used ''in-situ''. A mixture of both the ''cis'' (''Z-'') and ''trans'' (''E-'') isomers is produced. Both isomers are unstable, and they undergo a slow interconversion. The ''trans'' isomer is more stable, but the ''cis'' isomer is the one that reacts with unsaturated substrates, therefore the equilibrium between them sh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
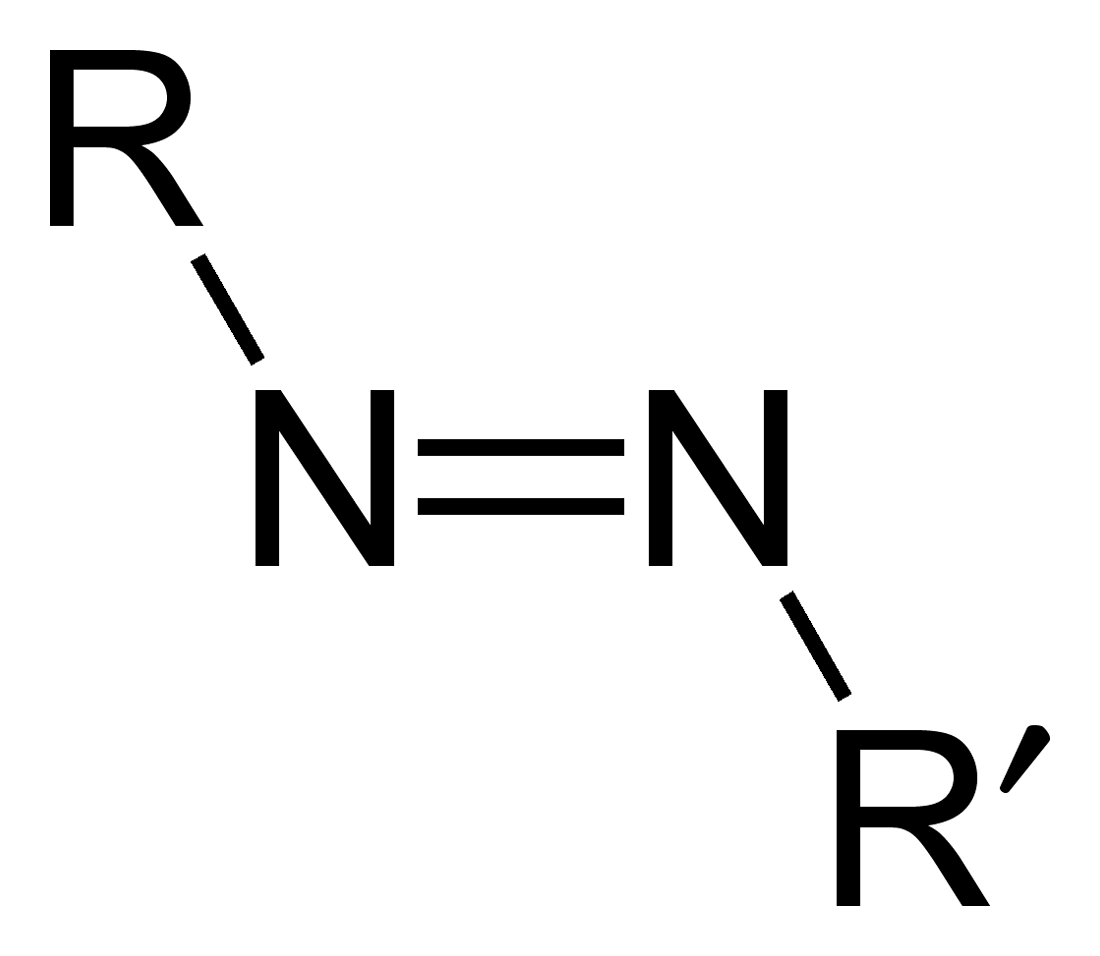
Azene
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups). IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted by hydrocarbyl groups, e.g. azobenzene or diphenyldiazene." The more stable derivatives contain two aryl groups. The group is called an ''azo group'' (, ). Many textile and leather articles are dyed with azo dyes and pigments. Aryl azo compounds Aryl azo compounds are usually stable, crystalline species. Azobenzene is the prototypical aromatic azo compound. It exists mainly as the Cis-trans isomerism, ''trans'' isomer, but upon illumination, converts to the Cis-trans isomerism, ''cis'' isomer. Aromatic azo compounds can be synthesized by azo coupling, which entails an electrophilic substitution reaction where an diazonium salt, aryl diazonium cation is attacked by another aryl ring, especially those substituted with electron-donating g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triazene
Triazene is an unsaturated inorganic compound having the chemical formula N3 H3. It has one double bond and is the second-simplest member of the azene class of hydronitrogen compounds, after diimide. Triazenes are a class of organic compounds containing the functional group -N(H)-N=N-. Triazene, possibly along with its isomer triimide (HNNHNH), has been synthesized in electron-irradiated ices of ammonia and ammonia/ dinitrogen and detected in the gas phase after sublimation.Forstel, Tsegaw, Maksyutenko, Mebel, Sander, & Kaiser. "On the formation of N3H3 isomers in irradiated ammonia bearing ices: Triazene (H2NNNH) or Triimide (HNHNNH)", ''ChemPhysChem'', 2016, 17, 2726. References External links *IUPAC Gold Book The International Union of Pure and Applied Chemistry publishes many books which contain its complete list of definitions. The definitions are divided into seven "colour books": Gold, Green, Blue, Purple, Orange, White, and Red. There is also an e ...br>definition ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrazene
Tetrazene is a chemical compound with the molecular formula H2NN=NNH2. It is a colorless explosive material. An analogue is the organosilicon derivative (tms)2NN=NN(tms)2 where tms is trimethylsilyl. Isomeric with tetrazine is ammonium azide. Tetrazene explosive, commonly known simply as tetrazene, is used for sensitization of priming compositions. Properties Tetrazene has eleven isomers. The most stable of these is the straight-chain 2-tetrazene (H2-NN=N-NH2), having a standard heat of formation at 301.3 kJ/mol. The eleven isomers can be arranged into three groups: straight-chain tetrazenes, four-membered cyclotetrazane, and three-membered cyclotriazanes. Each straight-chain tetrazene isomer possesses one N=N double bond and two N-N single bonds. Tautomerizations do occur between the isomers. The ionic compound ammonium azide is also a constitutional isomer of tetrazene. Organometallic derivatives A variety of coordination complex A coordination complex consists of a centr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia is both caust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geometric Isomer
Geometry (; ) is, with arithmetic, one of the oldest branches of mathematics. It is concerned with properties of space such as the distance, shape, size, and relative position of figures. A mathematician who works in the field of geometry is called a ''geometer''. Until the 19th century, geometry was almost exclusively devoted to Euclidean geometry, which includes the notions of point, line, plane, distance, angle, surface, and curve, as fundamental concepts. During the 19th century several discoveries enlarged dramatically the scope of geometry. One of the oldest such discoveries is Carl Friedrich Gauss' ("remarkable theorem") that asserts roughly that the Gaussian curvature of a surface is independent from any specific embedding in a Euclidean space. This implies that surfaces can be studied ''intrinsically'', that is, as stand-alone spaces, and has been expanded into the theory of manifolds and Riemannian geometry. Later in the 19th century, it appeared that geometri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organyl
In organic and organometallic chemistry, an organyl group is an organic substituent with one (sometimes more) free valence(-s) at a carbon atom.. The term is often used in chemical patent literature to protect claims over a broad scope. Examples * Acetonyl group * Acyl group (e.g. acetyl group, benzoyl group) * Alkyl group (e.g., methyl group, ethyl group) * Alkenyl group (e.g., vinyl group, allyl group) * Alkynyl group (propargyl group) * Benzyloxycarbonyl group (Cbz) * ''tert'' -butoxycarbonyl group (Boc) * Carboxyl group In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ... References Functional groups {{organic-chem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



-3D-balls.png)