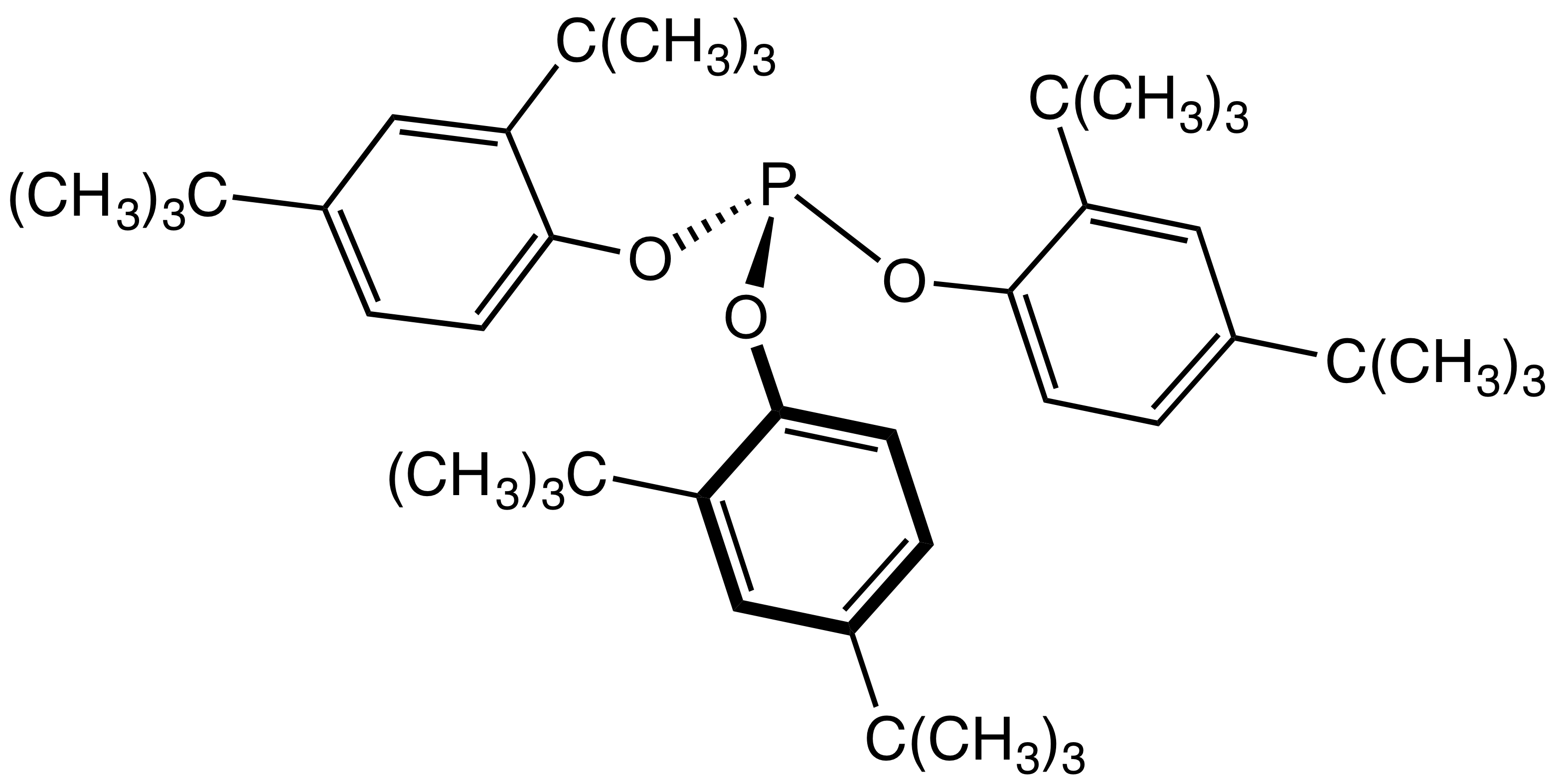
|
Diphenylphosphite
Diphenylphosphite is a diorganophosphite with the formula (C6H5O)2P(O)H. The molecule is tetrahedral. It is a colorless viscous liquid. The compounds can be prepared by treating phosphorus trichloride with phenol. Many analogues can be prepared similarly. One illustrative reaction, diphenylphosphite, aldehydes, and amines react to afford aminophosphonates (Kabachnik–Fields reaction).{{cite journal, title=An Extremely Efficient Three-Component Reaction of Aldehydes/Ketones, Amines, and Phosphites Kabachnik-Fields reaction for the Synthesis of α-Aminophosphonates Catalyzed by Magnesium Perchlorate, authors=Bhagat, Srikant; Chakraborti, Asit K., journal=Journal of Organic Chemistry, year=2007, volume=72, issue=4 , pages=1263–1270, doi=10.1021/jo062140i, pmid=17253748 See also *Dimethylphosphite *Diethylphosphite Diethylphosphite is the organophosphorus compound with the formula (C2H5O)2P(O)H. It is a popular reagent for generating other organophosphorus compounds, exploiting ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethylphosphite
Diethylphosphite is the organophosphorus compound with the formula (C2H5O)2P(O)H. It is a popular reagent for generating other organophosphorus compounds, exploiting the high reactivity of the P-H bond. Diethylphosphite is a colorless liquid. The molecule is tetrahedral. Synthesis and properties The compound was probably prepared in the 1850s by combining phosphorus trichloride and ethanol, but intentional preparations came later. It arises as follows: :PCl3 + 3 C2H5OH → (C2H5O)2P(O)H + 2 HCl + C2H5Cl Many derivatives can be prepared similarly. Despite being named as a phosphite it exists overwhelmingly in its phosphonate form, , a property it shares with its parent acid phosphorous acid; despite this many of its reactions are difficult to rationalise without assuming the existence of the following tautomerism equilibrium between phosphorus(V) and phosphorus(III) forms: :(C2H5O)2PV(O)H ⇌ (C2H5O)2PIII(OH) Reactions Alkoxide exchange Diethylphosphite undergoes transesterific ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphite Ester
The general structure of a phosphite ester showing the lone pairs on the P In organic chemistry, a phosphite ester or organophosphite usually refers to an organophosphorous compound with the formula P(OR)3. They can be considered as esters of an unobserved tautomer phosphorous acid, H3PO3, with the simplest example being trimethylphosphite, P(OCH3)3. Some phosphites can be considered esters of the dominant tautomer of phosphorous acid (HP(O)(OH)2). The simplest representative is dimethylphosphite with the formula HP(O)(OCH3)2. Both classes of phosphites are usually colorless liquids. Synthesis ;From PCl3 Phosphite esters are typically prepared by treating phosphorus trichloride with an alcohol. Depending on the synthetic details, this alcoholysis can give the diorganophosphites: :PCl3 + 3 C2H5OH → (C2H5O)2P(O)H + 2 HCl + C2H5Cl Alternatively, when the alcoholysis is conducted in the presence of proton acceptors, one obtains the C3-symmetric trialkoxy derivatives: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedral Molecular Geometry
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1(−) = 109.4712206...° ≈ 109.5° when all four substituents are the same, as in methane () as well as its heavier analogues. Methane and other perfectly symmetrical tetrahedral molecules belong to point group Td, but most tetrahedral molecules have lower symmetry. Tetrahedral molecules can be chiral. Tetrahedral bond angle The bond angle for a symmetric tetrahedral molecule such as CH4 may be calculated using the dot product of two vectors. As shown in the diagram, the molecule can be inscribed in a cube with the tetravalent atom (e.g. carbon) at the cube centre which is the origin of coordinates, O. The four monovalent atoms (e.g. hydrogens) are at four corners of the cube (A, B, C, D) chosen so that no two atoms are at adjacent corners linked by only one cube edge. If the edge length of the cube ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Trichloride
Phosphorus trichloride is an inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic and reacts readily with water to release hydrogen chloride. History Phosphorus trichloride was first prepared in 1808 by the French chemists Joseph Louis Gay-Lussac and Louis Jacques Thénard by heating calomel (Hg2Cl2) with phosphorus. Later during the same year, the English chemist Humphry Davy produced phosphorus trichloride by burning phosphorus in chlorine gas. Preparation World production exceeds one-third of a million tonnes. Phosphorus trichloride is prepared industrially by the reaction of chlorine with white phosphorus, using phosphorus trichloride as the solvent. In this continuous process PCl3 is removed as it is formed in order to avoid the formation of PCl5. :P4 + 6 Cl2 → 4 PCl3 Structure and spectroscopy It has a trig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aminophosphonate
Aminophosphonates are organophosphorus compounds with the formula (RO)2P(O)CR'2NR"2. These compounds are structural analogues of amino acids in which a carboxylic moiety is replaced by phosphonic acid or related groups. Acting as antagonists of amino acids, they inhibit enzymes involved in amino acid metabolism and thus affect the physiological activity of the cell. These effects may be exerted as antibacterial, plant growth regulatory or neuromodulatory. They can act as ligands, and heavy metal complexes with aminophosphonates have had medical applications investigated. Phosphonates are more difficult to hydrolyse than phosphates. Preparation Aminophosphonates are often prepared by hydrophosphonylation, usually the condensation of imines and phosphorous acid. In the Pudovik reaction or Kabachnik–Fields reaction the esters of phosphorous acid are employed, e.g. diphenylphosphite. Because these compounds are of pharmaceutical interest, methods have been developed to induce the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kabachnik–Fields Reaction
In organophosphorus chemistry, the Kabachnik–Fields reaction is a three-component organic reaction forming aminophosphonate, α-aminomethylphosphonates from an amine, a carbonyl compound, and a phosphite ester, dialkyl phosphonate, (RO)2P(O)H (that are also called dialkylphosphites). Aminophosphonates are synthetic targets of some importance as phosphorus analog (chemistry), analogues of α-amino acids (a bioisosterism, bioisostere). This multicomponent reaction was independently discovered by and Ellis K. Fields in 1952. The reaction is very similar to the two-component Pudovik reaction, which involves condensation of the phosphite and a preformed imine. : The first step in this reaction is the formation of an imine, followed by a hydrophosphonylation step where the phosphonate P-H bond across the C=N double bond. The starting carbonyl component is usually an aldehyde and sometimes a ketone. The reaction can be accelerated with a combination of dehydrating reagent and Lewis acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylphosphite
Dimethyl hydrogen phosphite (DMHP), also known as Dimethylphosphite, is an organophosphorus compound with the formula (CH3O)2P(O)H. It is a reagent for generating other organophosphorus compounds, exploiting the high reactivity of the P-H bond. The molecule is tetrahedral. It is a colorless liquid. The compounds can be prepared by methanolysis of phosphorus trichloride or by heating diethylphosphite in methanol. Due to the presence of hydrogen, a "soft" ligand, the compound resonates. DMHP exists in chemical equilibrium, in two structures. One of the structures has a lone electron cloud, which is nucleophilically attacking the remaining tetrahedral structure. Due to the structural equilibrium tending towards the phosphonate, this reaction is slow, needing a chemical or electromagnetic catalyst (heat). This tautomeric nature of DMHP made it desirable as a precursor to the G-series compounds, and it was the most successful among all other phosphonate precursors. The now obsolete ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diisopropylphosphite
Diisopropylphosphite is an organophosphorus compound with the formula (i-PrO)2P(O)H (i-Pr = CH(CH3)2). The molecule is tetrahedral. It is a colorless viscous liquid. The compounds can be prepared by treating phosphorus trichloride with isopropanol Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable organic compound with a pungent alcoholic odor. As an isopropyl group linked to a hydroxyl group (chemical formula ) it is the simple ....{{cite journal, last = Pedrosa, first = Leandro, year = 2011, url = http://cssp.chemspider.com/488, title = Esterification of Phosphorus Trichloride with Alcohols; Diisopropyl phosphonate, journal = ChemSpider Synthetic Pages, pages=488, doi = 10.1039/SP488, publisher = Royal Society of Chemistry, doi-access = free References Organophosphites ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |