|
Dillapiol
Dillapiole is an organic chemical compound and essential oil commonly extracted from dill weed, though it can be found in a variety of other plants such as fennel root. This compound is closely related to apiole, having a methoxy group positioned differently on the benzene ring. Dillapiole works synergically with certain insecticides like pyrethrins similarly to piperonyl butoxide, which likely results from inhibition of the MFO enzyme of insects. No carcinogenicity was detected with parsley apiol or dill apiol in mice. References See also * Apiole * Phenylpropene Phenylpropene is the organic compound with the formula C6H5CH2CH=CH2. It is a colorless liquid. The compound consists of a phenyl group attached to allyl. Phenylpropene isomerizes to trans-propenylbenzene. In plant biochemistry, the phenylpro ... Phenylpropenes O-methylated phenylpropanoids Benzodioxoles Allyl compounds Pyrogallol ethers Hydroxyquinol ethers {{aromatic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apiole
Apiole is a phenylpropene, also known as apiol, parsley apiol, or parsley camphor. Its chemical name is 1-allyl-2,5-dimethoxy-3,4-methylenedioxybenzene. It is found in the essential oils of celery leaf and all parts of parsley. Heinrich Christoph Link, an apothecary in Leipzig, discovered the substance in 1715 as greenish crystals reduced by steam from oil of parsley. In 1855 Joret and Homolle discovered that ''apiol'' was an effective treatment of amenorrea or lack of menstruation. In medicine it has been used, as essential oil or in purified form, for the treatment of menstrual disorders and as an abortifacient. It is an irritant and, in high doses, it can cause liver and kidney damage. Cases of death due to attempted abortion using apiole have been reported. Hippocrates wrote about parsley as an herb to cause an abortion. Plants containing apiole were used by women in the Middle Ages to terminate pregnancies. Now that safer methods of abortion are available, apiol is almost fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apiole
Apiole is a phenylpropene, also known as apiol, parsley apiol, or parsley camphor. Its chemical name is 1-allyl-2,5-dimethoxy-3,4-methylenedioxybenzene. It is found in the essential oils of celery leaf and all parts of parsley. Heinrich Christoph Link, an apothecary in Leipzig, discovered the substance in 1715 as greenish crystals reduced by steam from oil of parsley. In 1855 Joret and Homolle discovered that ''apiol'' was an effective treatment of amenorrea or lack of menstruation. In medicine it has been used, as essential oil or in purified form, for the treatment of menstrual disorders and as an abortifacient. It is an irritant and, in high doses, it can cause liver and kidney damage. Cases of death due to attempted abortion using apiole have been reported. Hippocrates wrote about parsley as an herb to cause an abortion. Plants containing apiole were used by women in the Middle Ages to terminate pregnancies. Now that safer methods of abortion are available, apiol is almost fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemical Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon chemical bond, bonds. Due to carbon's ability to Catenation, catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate, carbonate salts and cyanide, cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered Inorganic chemistry, inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allyl Compounds
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "". The term allyl applies to many compounds related to , some of which are of practical or of everyday importance, for example, allyl chloride. Allylation is any chemical reaction that adds an allyl group to a substrate. Nomenclature A site adjacent to the unsaturated carbon atom is called the allylic position or allylic site. A group attached at this site is sometimes described as allylic. Thus, "has an allylic hydroxyl group". Allylic C−H bonds are about 15% weaker than the C−H bonds in ordinary sp3 carbon centers and are thus more reactive. Benzylic and allylic are related in terms of structure, bond strength, and reactivity. Other re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
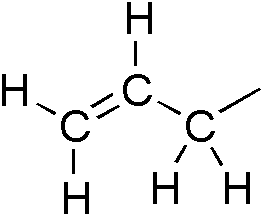
Phenylpropenes
Phenylpropene is the organic compound with the formula C6H5CH2CH=CH2. It is a colorless liquid. The compound consists of a phenyl group attached to allyl. Phenylpropene isomerizes to trans-propenylbenzene. In plant biochemistry, the phenylpropene skeleton is the parent (simplest representation) of the phenylpropanoids. Prominent derivatives include eugenol, safrole Safrole is an organic compound with the formula CH2O2C6H3CH2CH=CH2. It is a colorless oily liquid, although impure samples can appear yellow. A member of the phenylpropanoid family of natural products, it is found in sassafras plants, among oth ..., and many others. References External links * {{Phenylpropene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylpropene
Phenylpropene is the organic compound with the formula C6H5CH2CH=CH2. It is a colorless liquid. The compound consists of a phenyl group attached to allyl. Phenylpropene isomerizes to trans-propenylbenzene. In plant biochemistry, the phenylpropene skeleton is the parent (simplest representation) of the phenylpropanoids. Prominent derivatives include eugenol, safrole Safrole is an organic compound with the formula CH2O2C6H3CH2CH=CH2. It is a colorless oily liquid, although impure samples can appear yellow. A member of the phenylpropanoid family of natural products, it is found in sassafras plants, among oth ..., and many others. References External links * {{Phenylpropene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Piperonyl Butoxide
Piperonyl butoxide (PBO) is a pale yellow to light brown liquidNational Toxicology Program, Institute of Environmental Health Sciences, National Institutes of Health (NTP). 1992. National Toxicology Program Chemical Repository Database. Research Triangle Park, North Carolina. organic compound used as a synergist component of pesticide formulations. That is, despite having no pesticidal activity of its own, it enhances the potency of certain pesticides such as carbamates, pyrethrins, pyrethroids, and rotenone. It is a semisynthetic derivative of safrole.Robert L. Metcalf "Insect Control" in Ullmann’s Encyclopedia of Industrial Chemistry" Wiley-VCH, Weinheim, 2002. History PBO was developed in the late 1930s and early 1940s to enhance the performance of the naturally derived insecticide pyrethrum. Pyrethrum is and was an important insecticide against mosquitoes and other disease-carrying vectors, thereby providing public health benefits, e.g., preventing malaria. Although exhibi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mixed-function Oxidase
Mixed-function oxidase is the name of a family of oxidase enzymes that catalyze a reaction in which each of the two atoms of oxygen in O2 is used for a different function in the reaction. Oxidase is a general name for enzymes that catalyze oxidations in which molecular oxygen is the electron acceptor but oxygen atoms do not appear in the oxidized product. Often, oxygen is reduced to either water (cytochrome oxidase of the mitochondrial electron transfer chain) or hydrogen peroxide (dehydrogenation of fatty acyl-CoA in peroxisomes). Most of the oxidases are flavoprotein Flavoproteins are proteins that contain a nucleic acid derivative of riboflavin. Flavoproteins are involved in a wide array of biological processes, including removal of radicals contributing to oxidative stress, photosynthesis, and DNA repair. T ...s. The name "mixed-function oxidase" indicates that the enzyme oxidizes two different substrate simultaneously. Desaturation of fatty acyl-CoA in vertebrates is an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Essential Oil
An essential oil is a concentrated hydrophobic liquid containing volatile (easily evaporated at normal temperatures) chemical compounds from plants. Essential oils are also known as volatile oils, ethereal oils, aetheroleum, or simply as the oil of the plant from which they were extracted, such as oil of clove. An essential oil is essential in the sense that it contains the essence of the plant's fragrance—the characteristic fragrance of the plant from which it is derived. The term "essential" used here does ''not'' mean indispensable or usable by the human body, as with the terms essential amino acid or essential fatty acid, which are so called because they are nutritionally required by a living organism. Essential oils are generally extracted by distillation, often by using steam. Other processes include expression, solvent extraction, '' sfumatura'', absolute oil extraction, resin tapping, wax embedding, and cold pressing. They are used in perfumes, cosmetics, soaps, air ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrethrin
The pyrethrins are a class of organic compounds normally derived from ''Chrysanthemum cinerariifolium'' that have potent Insecticide, insecticidal activity by targeting the nervous systems of insects. Pyrethrin naturally occurs in chrysanthemum flowers and is often considered an organic horticulture, organic insecticide when it is not combined with piperonyl butoxide or other synthetic agricultural spray adjuvant, adjuvants.Mader, Eric, and Nancy Lee Adamson. "Organic-Approved Pesticides."Organic-Approved Pesticides (n.d.): n. pag. The Xerxes Society. The Xerces Society for Invertebrate Conservation, Oct. 2012. Web. 10 Mar. 2015. Their insecticidal and insect-repellent properties have been known and used for thousands of years. Pyrethrins are gradually replacing organophosphates and organochlorides as the pesticides of choice as the latter compounds have been shown to have significant and persistent toxic effects to humans.They first appeared on markets in the 1900's and have bee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |