|
Diffusion Pressure Deficit
Suction pressure is also called Diffusion Pressure Deficit. If some solute is dissolved in solvent, its diffusion pressure decreases. The difference between diffusion pressure of pure solvent and solution is called diffusion pressure deficit (DPD). It is a reduction in the diffusion pressure of solvent in the solution over its pure state due to the presence of solutes in it and forces opposing diffusion. When a plant cell is placed in a hypotonic solution, water enters into a cell by endosmosis and as a result turgor pressure (TP) develops in the cell. The cell membrane becomes stretched and the osmotic pressure (OP) of the cell decreases. As the cell absorbs more and more water its turgor pressure increases and osmotic pressure decreases. When a cell is fully turgid, its OP is equal to TP and DPD is zero. Turgid cells cannot absorb any more water. Thus, with reference to plant cells, the DPD can be described as the actual thirst of a cell for water and can be express ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypotonic
In chemical biology, tonicity is a measure of the effective osmotic pressure gradient; the water potential of two solutions separated by a partially-permeable cell membrane. Tonicity depends on the relative concentration of selective membrane-impermeable solutes across a cell membrane which determine the direction and extent of osmotic flux. It is commonly used when describing the swelling-versus-shrinking response of cells immersed in an external solution. Unlike osmotic pressure, tonicity is influenced only by solutes that cannot cross the membrane, as only these exert an effective osmotic pressure. Solutes able to freely cross the membrane do not affect tonicity because they will always equilibrate with equal concentrations on both sides of the membrane without net solvent movement. It is also a factor affecting imbibition. There are three classifications of tonicity that one solution can have relative to another: ''hypertonic'', ''hypotonic'', and ''isotonic''.A hypotonic so ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
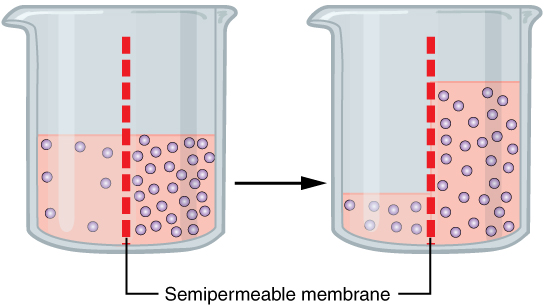
Endosmosis
Osmosis (, ) is the spontaneous net movement or diffusion of solvent molecules through a selectively-permeable membrane from a region of high water potential (region of lower solute concentration) to a region of low water potential (region of higher solute concentration), in the direction that tends to equalize the solute concentrations on the two sides. It may also be used to describe a physical process in which any solvent moves across a selectively permeable membrane (permeable to the solvent, but not the solute) separating two solutions of different concentrations. Osmosis can be made to do work. Osmotic pressure is defined as the external pressure required to be applied so that there is no net movement of solvent across the membrane. Osmotic pressure is a colligative property, meaning that the osmotic pressure depends on the molar concentration of the solute but not on its identity. Osmosis is a vital process in biological systems, as biological membranes are semipermeab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Turgor Pressure
Turgor pressure is the force within the cell that pushes the plasma membrane against the cell wall. It is also called ''hydrostatic pressure'', and is defined as the pressure in a fluid measured at a certain point within itself when at equilibrium. Generally, turgor pressure is caused by the osmotic flow of water and occurs in plants, fungi, and bacteria. The phenomenon is also observed in protists that have cell walls. This system is not seen in animal cells, as the absence of a cell wall would cause the cell to lyse when under too much pressure. The pressure exerted by the osmotic flow of water is called turgidity. It is caused by the osmotic flow of water through a selectively permeable membrane. Movement of water through a semipermeable membrane from a volume with a low solute concentration to one with a higher solute concentration is called osmotic flow. In plants, this entails the water moving from the low concentration solute outside the cell into the cell's vacuole. Mec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osmotic Pressure
Osmotic pressure is the minimum pressure which needs to be applied to a solution to prevent the inward flow of its pure solvent across a semipermeable membrane. It is also defined as the measure of the tendency of a solution to take in a pure solvent by osmosis. Potential osmotic pressure is the maximum osmotic pressure that could develop in a solution if it were separated from its pure solvent by a semipermeable membrane. Osmosis occurs when two solutions containing different concentrations of solute are separated by a selectively permeable membrane. Solvent molecules pass preferentially through the membrane from the low-concentration solution to the solution with higher solute concentration. The transfer of solvent molecules will continue until equilibrium is attained. Theory and measurement Jacobus van 't Hoff found a quantitative relationship between osmotic pressure and solute concentration, expressed in the following equation: :\Pi = icRT where \Pi is osmotic p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Otto Renner
Otto Renner (25 April 1883 in Neu-Ulm – 8 July 1960) was a German plant geneticist. Following the work of Erwin Baur, Renner established the theory of maternal plastid inheritance as a widely accepted genetic theory. He studied botany under Karl von Goebel and Ludwig Radlkofer at the University of Munich, and with Wilhelm Pfeffer at the University of Leipzig. From 1913 to 1920 he served as an associate professor of plant physiology at Munich, and afterwards, succeeded Christian Ernst Stahl as chair of botany at the University of Jena, where he was also director of the botanical gardens. In 1946 he returned as a professor to the University of Munich. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Refrigeration
The term refrigeration refers to the process of removing heat from an enclosed space or substance for the purpose of lowering the temperature.International Dictionary of Refrigeration, http://dictionary.iifiir.org/search.phpASHRAE Terminology, https://www.ashrae.org/technical-resources/free-resources/ashrae-terminology Refrigeration can be considered an artificial, or human-made, cooling method. Refrigeration refers to the process by which energy, in the form of heat, is removed from a low-temperature medium and transferred to a high-temperature medium. This work of energy transfer is traditionally driven by mechanical means, but can also be driven by heat, magnetism, electricity, laser, or other means. Refrigeration has many applications, including household refrigerators, industrial freezers, cryogenics, and air conditioning. Heat pumps may use the heat output of the refrigeration process, and also may be designed to be reversible, but are otherwise similar to air conditioning ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Air Conditioning
Air conditioning, often abbreviated as A/C or AC, is the process of removing heat from an enclosed space to achieve a more comfortable interior environment (sometimes referred to as 'comfort cooling') and in some cases also strictly controlling the humidity of internal air. Air conditioning can be achieved using a mechanical 'air conditioner' or alternatively a variety of other methods, including passive cooling or ventilative cooling. Air conditioning is a member of a family of systems and techniques that provide heating, ventilation, and air conditioning (HVAC). Heat pumps are similar in many ways to air conditioners, but use a reversing valve to allow them to both heat and also cool an enclosed space. Air conditioners, which typically use vapor-compression refrigeration, range in size from small units used within vehicles or single rooms to massive units that can cool large buildings. Air source heat pumps, which can be used for heating as well as cooling, are becoming incre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wet Bulb Temperature
The wet-bulb temperature (WBT) is the temperature read by a thermometer covered in water-soaked (water at ambient temperature) cloth (a wet-bulb thermometer) over which air is passed. At 100% relative humidity, the wet-bulb temperature is equal to the air temperature (dry-bulb temperature); at lower humidity the wet-bulb temperature is lower than dry-bulb temperature because of evaporative cooling. The wet-bulb temperature is defined as the temperature of a parcel of air cooled to saturation (100% relative humidity) by the evaporation of water into it, with the latent heat supplied by the parcel. A wet-bulb thermometer indicates a temperature close to the true (thermodynamic) wet-bulb temperature. The wet-bulb temperature is the lowest temperature that can be reached under current ambient conditions by the evaporation of water only. Even heat-adapted people cannot carry out normal outdoor activities past a wet-bulb temperature of , equivalent to a heat index of . The theoretic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |