|
Dibutyl Maleate
Dibutyl maleate is an organic compound with the formula (CHCO2Bu)2 (Bu = butyl). It is the diester of the unsaturated dicarboxylic acid maleic acid. It is a colorless oily liquid, although impure samples can appear yellow. Preparation Dibutyl maleate can be prepared by the reaction of maleic acid anhydride and 1-butanol in presence of p-toluenesulfonic acid. Uses Dibutyl maleate is mainly used as a plasticizer for aqueous dispersions of copolymers with vinyl acetate and as an intermediate in the preparation of other chemical compounds. With the invention of polyaspartic technology the material found another use. In this situation, an amine is reacted with a dialkyl maleate - usually diethyl maleate but also dibutyl maleate may be used- utilizing the Michael addition reaction. The resulting products, polyaspartic esters products are then used in coatings, adhesives, sealants and elastomers An elastomer is a polymer with viscoelasticity (i.e. both viscosity and elasticity ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Living t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
OECD
The Organisation for Economic Co-operation and Development (OECD; french: Organisation de coopération et de développement économiques, ''OCDE'') is an intergovernmental organisation with 38 member countries, founded in 1961 to stimulate economic progress and world trade. It is a forum whose member countries describe themselves as committed to democracy and the market economy, providing a platform to compare policy experiences, seek answers to common problems, identify good practices, and coordinate domestic and international policies of its members. The majority of OECD members are high-income economies with a very high Human Development Index (HDI), and are regarded as developed countries. Their collective population is 1.38 billion. , the OECD member countries collectively comprised 62.2% of global nominal GDP (US$49.6 trillion) and 42.8% of global GDP ( Int$54.2 trillion) at purchasing power parity. The OECD is an official United Nations observer. In April 1948, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethyl Maleate
Dimethyl maleate is an organic compound with the formula C6H8O4. It is the dimethyl ester of maleic acid. Synthesis Dimethyl maleate can be synthesized from maleic anhydride and methanol, with sulfuric acid acting as acid catalyst, via a nucleophilic acyl substitution for the monomethyl ester, followed by a Fischer esterification reaction for the dimethyl ester. Applications Dimethyl maleate is used in many organic syntheses as a dienophile for diene synthesis. It is used as an additive and intermediate for plastics, pigments, pharmaceuticals, and agricultural products. It is also an intermediate for the production of paints, adhesives, and copolymers. Dimethyl maleate has also found use in applications where improvements in the hardness and toughness of polymer films are desired. This includes, in particular, the improvement of anti-blocking properties of copolymers of vinyl acetate with DMM. It is also used as an internal modifier to increase the glass transition temperature o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethyl Maleate
Diethyl maleate is an organic compound with the CAS Registry number 141-05-9. It is chemically a maleate ester with the formula C8H12O4. It is a colorless liquid at room temperature. It has the IUPAC name of diethyl (''Z'')-but-2-enedioate. Synthesis The material is synthesized by the esterification of maleic acid or maleic anhydride and ethanol. Uses One of the key uses for the compound is in production of the pesticide Malathion. It has also been used medically as a chemical depletory of glutathione. It has been studied extensively with regard to renal function. Other medical uses include treatment of breast cancer and its monitoring with Positron Emission Tomography. It is also used as a food additive and has Food and Drug Administration clearance for indirect food contact. In synthetic organic chemistry it is a dienophile and used in the Diels-Alder reaction. With the invention of polyaspartic technology the material also found another use. With this technology an amine i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elastomers
An elastomer is a polymer with viscoelasticity (i.e. both viscosity and elasticity) and with weak intermolecular forces, generally low Young's modulus and high failure strain compared with other materials. The term, a portmanteau of ''elastic polymer'', is often used interchangeably with rubber, although the latter is preferred when referring to vulcanisates. Each of the monomers which link to form the polymer is usually a compound of several elements among carbon, hydrogen, oxygen and silicon. Elastomers are amorphous polymers maintained above their glass transition temperature, so that considerable molecular reconformation is feasible without breaking of covalent bonds. At ambient temperatures, such rubbers are thus relatively compliant ( E ≈ 3 M Pa) and deformable. Their primary uses are for seals, adhesives and molded flexible parts. Application areas for different types of rubber are manifold and cover segments as diverse as tires, soles for shoes, and damping and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
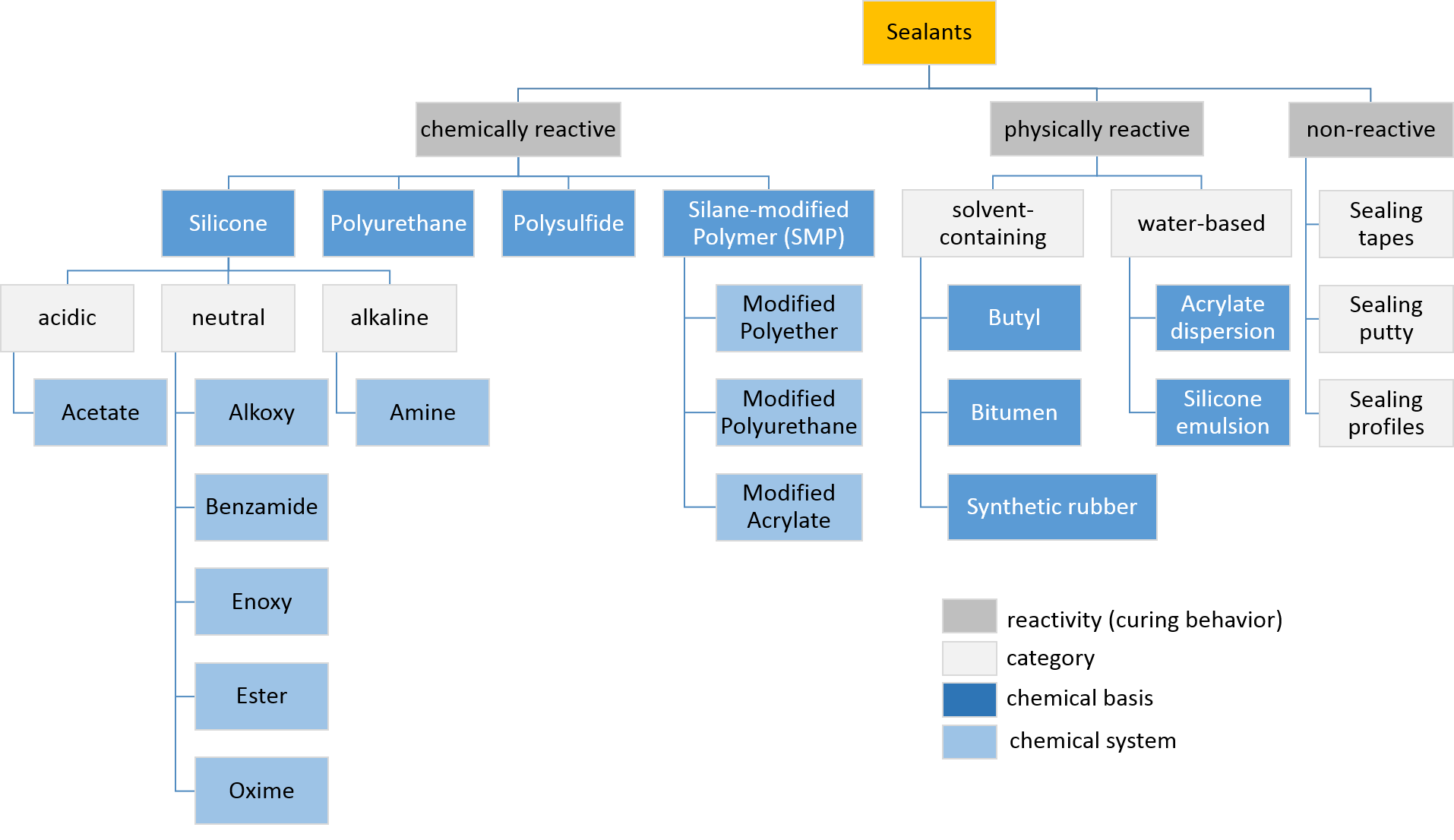
Sealants
Sealant is a substance used to block the passage of fluids through openings in materials, a type of mechanical seal. In building construction ''sealant'' is sometimes synonymous with ''caulking'' and also serve the purposes of blocking dust, sound and heat transmission. Sealants may be weak or strong, flexible or rigid, permanent or temporary. Sealants are not adhesives but some have adhesive qualities and are called ''adhesive-sealants'' or ''structural sealants''. History Sealants were first used in prehistory in the broadest sense as mud, grass and reeds to seal dwellings from the weather such as the daub in wattle and daub and thatching. Natural sealants and adhesive-sealants included plant resins such as pine pitch and birch pitch, bitumen, wax, tar, natural gum, clay (mud) mortar, lime mortar, lead, blood and egg. In the 17th century glazing putty was first used to seal window glass made with linseed oil and chalk, later other drying oils were also used to make oil-based ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adhesives
Adhesive, also known as glue, cement, mucilage, or paste, is any non-metallic substance applied to one or both surfaces of two separate items that binds them together and resists their separation. The use of adhesives offers certain advantages over other binding techniques such as sewing, mechanical fastenings, or welding. These include the ability to bind different materials together, the more efficient distribution of stress across a joint, the cost-effectiveness of an easily mechanized process, and greater flexibility in design. Disadvantages of adhesive use include decreased stability at high temperatures, relative weakness in bonding large objects with a small bonding surface area, and greater difficulty in separating objects during testing. Adhesives are typically organized by the method of adhesion followed by ''reactive'' or ''non-reactive'', a term which refers to whether the adhesive chemically reacts in order to harden. Alternatively, they can be organized eithe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coatings
A coating is a covering that is applied to the surface of an object, usually referred to as the substrate. The purpose of applying the coating may be decorative, functional, or both. Coatings may be applied as liquids, gases or solids e.g. Powder coatings. Paints and lacquers are coatings that mostly have dual uses of protecting the substrate and being decorative, although some artists paints are only for decoration, and the paint on large industrial pipes is for preventing corrosion and identification e.g. blue for process water, red for fire-fighting control etc. Functional coatings may be applied to change the surface properties of the substrate, such as adhesion, wettability, corrosion resistance, or wear resistance. In other cases, e.g. semiconductor device fabrication (where the substrate is a wafer), the coating adds a completely new property, such as a magnetic response or electrical conductivity, and forms an essential part of the finished product. A major consideratio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Michael Addition
In organic chemistry, the Michael reaction or Michael addition is a reaction between a Michael donor (an enolate or other nucleophile) and a Michael acceptor (usually an α,β-unsaturated carbonyl) to produce a Michael adduct by creating a carbon-carbon bond at the acceptor's β-carbon. It belongs to the larger class of conjugate additions and is widely used for the mild formation of carbon-carbon bonds. The Michael addition is an important atom-economical method for diastereoselective and enantioselective C–C bond formation, and many asymmetric variants exist : In this general Michael addition scheme, either or both of R and R' on the nucleophile (the Michael donor) represent electron-withdrawing substituents such as acyl, cyano, nitro, or sulfone groups, which make the adjacent methylene hydrogen acidic enough to form a carbanion when reacted with the base, ''B:''. For the alkene (the Michael acceptor), the R" substituent is usually a carbonyl, which makes the compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyaspartic
Polyaspartic ester chemistry was first introduced in the early 1990s making it a relatively new technology. The patents were issued to Bayer in Germany and Miles Corporation in the United States. It utilizes the aza-Michael addition reaction. These products are then used in coatings, adhesives, sealants and elastomers. Pure polyurea reacts extremely quickly making them almost unusable without plural component spray equipment. Polyaspartic technology utilizes a partially blocked amine to react more slowly with the isocyanates and thus produce a modified polyurea. The amine/diamine or even triamine functional coreactant for aliphatic polyisocyanate is typically reacted with a maleate. Polyaspartic esters (PAE) initially found use in conventional solvent-borne two-component polyurethane coatings. Chemistry To manufacture a polyaspartic ester, an amine is reacted with dialkyl maleate by the aza-Michael reaction. Diethyl maleate is the usual maleate used. This converts the primary ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinyl Acetate
Vinyl acetate is an organic compound with the formula CH3CO2CH=CH2. This colorless liquid is the precursor to polyvinyl acetate and ethene-vinyl acetate copolymers, important industrial polymers. Production The worldwide production capacity of vinyl acetate was estimated at 6,969,000 tonnes/year in 2007, with most capacity concentrated in the United States (1,585,000 all in Texas), China (1,261,000), Japan (725,000) and Taiwan (650,000). The average list price for 2008 was US$1600/tonne. Celanese is the largest producer (ca 25% of the worldwide capacity), while other significant producers include China Petrochemical Corporation (7%), Chang Chun Group (6%), and LyondellBasell (5%). It is a key ingredient in furniture glue. Preparation Vinyl acetate is the acetate ester of vinyl alcohol. Since vinyl alcohol is highly unstable (with respect to acetaldehyde), the preparation of vinyl acetate is more complex than the synthesis of other acetate esters. The major industrial route i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula , derived from either of the two isomers (''n''-butane and isobutane) of butane. The isomer ''n''-butane can connect in two ways, giving rise to two "-butyl" groups: * If it connects at one of the two terminal carbon atoms, it is normal butyl or ''n''-butyl: (preferred IUPAC name: butyl) * If it connects at one of the non-terminal (internal) carbon atoms, it is secondary butyl or ''sec''-butyl: (preferred IUPAC name: butan-2-yl) The second isomer of butane, isobutane, can also connect in two ways, giving rise to two additional groups: * If it connects at one of the three terminal carbons, it is isobutyl: (preferred IUPAC name: 2-methylpropyl) * If it connects at the central carbon, it is tertiary butyl, ''tert''-butyl or ''t''-butyl: (preferred IUPAC name: ''tert''-butyl) Nomenclature According to IUPAC nomenclature, "isobutyl", "''sec''-butyl", and "''tert''-b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |