|
Diathermal Wall
In thermodynamics, a diathermal wall between two thermodynamic systems allows heat transfer but does not allow transfer of matter across it. The diathermal wall is important because, in thermodynamics, it is customary to assume ''a priori'', for a closed system, the physical existence of transfer of energy across a wall that is impermeable to matter but is not adiabatic, transfer which is called transfer of energy as heat, though it is not customary to label this assumption separately as an axiom or numbered law. Definitions of transfer of heat In theoretical thermodynamics, respected authors vary in their approaches to the definition of quantity of heat transferred. There are two main streams of thinking. One is from a primarily empirical viewpoint (which will here be referred to as the thermodynamic stream), to define heat transfer as occurring only by specified macroscopic mechanisms; loosely speaking, this approach is historically older. The other (which will here be referred ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering and mechanical engineering, but also in other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the efficiency of early steam engines, particularly through the work of French physicist Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win the Napoleonic Wars. Scots-Irish physicist Lord Kelvin was the first to formulate a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
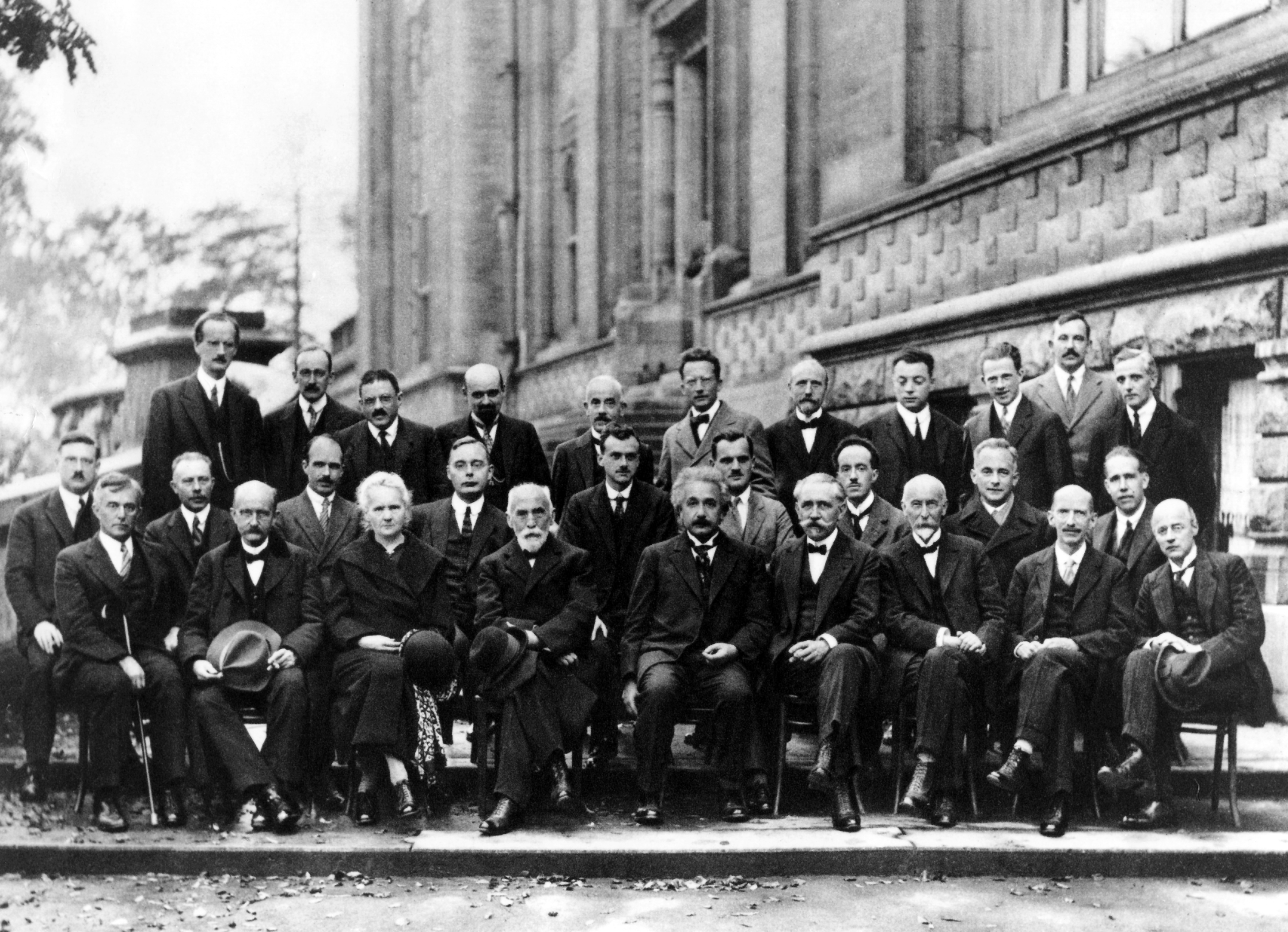
Max Planck
Max Karl Ernst Ludwig Planck (, ; 23 April 1858 – 4 October 1947) was a German theoretical physicist whose discovery of energy quanta won him the Nobel Prize in Physics in 1918. Planck made many substantial contributions to theoretical physics, but his fame as a physicist rests primarily on his role as the originator of quantum theory, which revolutionized human understanding of atomic and subatomic processes. In 1948, the German scientific institution Kaiser Wilhelm Society (of which Planck was twice president) was renamed Max Planck Society (MPG). The MPG now includes 83 institutions representing a wide range of scientific directions. Life and career Planck came from a traditional, intellectual family. His paternal great-grandfather and grandfather were both theology professors in Göttingen; his father was a law professor at the University of Kiel and Munich. One of his uncles was also a judge. Planck was born in 1858 in Kiel, Holstein, to Johann Julius Wilhelm Plan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
John Gamble Kirkwood
John "Jack" Gamble Kirkwood (May 30, 1907, Gotebo, Oklahoma – August 9, 1959, New Haven, Connecticut) was a noted chemist and physicist, holding faculty positions at Cornell University, the University of Chicago, California Institute of Technology, and Yale University. Early life and background Kirkwood was born in Gotebo, Oklahoma, the oldest child of John Millard and Lillian Gamble Kirkwood. His father was educated as an attorney and was a distributor for the Goodyear Corporation in the state of Kansas. In addition to Jack Kirkwood, there were two younger sisters: Caroline (1910) and Margaret (1921). In 1909, the family moved to Wichita, Kansas. In the 1920s the family traveled to Pasadena, California to escape Midwestern winters. Education While in Pasadena, Kirkwood, age 15, audited chemistry classes at Caltech. Showing remarkable talent in mathematics and chemistry, Kirkwood was persuaded by A. A. Noyes to enroll at Caltech before finishing his high school education ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elsevier
Elsevier () is a Dutch academic publishing company specializing in scientific, technical, and medical content. Its products include journals such as ''The Lancet'', ''Cell'', the ScienceDirect collection of electronic journals, '' Trends'', the '' Current Opinion'' series, the online citation database Scopus, the SciVal tool for measuring research performance, the ClinicalKey search engine for clinicians, and the ClinicalPath evidence-based cancer care service. Elsevier's products and services also include digital tools for data management, instruction, research analytics and assessment. Elsevier is part of the RELX Group (known until 2015 as Reed Elsevier), a publicly traded company. According to RELX reports, in 2021 Elsevier published more than 600,000 articles annually in over 2,700 journals; as of 2018 its archives contained over 17 million documents and 40,000 e-books, with over one billion annual downloads. Researchers have criticized Elsevier for its high profit marg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
George H
George may refer to: People * George (given name) * George (surname) * George (singer), American-Canadian singer George Nozuka, known by the mononym George * George Washington, First President of the United States * George W. Bush, 43rd President of the United States * George H. W. Bush, 41st President of the United States * George V, King of Great Britain, Ireland, the British Dominions and Emperor of India from 1910-1936 * George VI, King of Great Britain, Ireland, the British Dominions and Emperor of India from 1936-1952 * Prince George of Wales * George Papagheorghe also known as Jorge / GEØRGE * George, stage name of Giorgio Moroder * George Harrison, an English musician and singer-songwriter Places South Africa * George, Western Cape ** George Airport United States * George, Iowa * George, Missouri * George, Washington * George County, Mississippi * George Air Force Base, a former U.S. Air Force base located in California Characters * George (Peppa Pig), a 2-year-old ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Max Born
Max Born (; 11 December 1882 – 5 January 1970) was a German physicist and mathematician who was instrumental in the development of quantum mechanics. He also made contributions to solid-state physics and optics and supervised the work of a number of notable physicists in the 1920s and 1930s. Born won the 1954 Nobel Prize in Physics for his "fundamental research in quantum mechanics, especially in the statistical interpretation of the wave function". Born entered the University of Göttingen in 1904, where he met the three renowned mathematicians Felix Klein, David Hilbert, and Hermann Minkowski. He wrote his PhD thesis on the subject of "Stability of Elastica in a Plane and Space", winning the university's Philosophy Faculty Prize. In 1905, he began researching special relativity with Minkowski, and subsequently wrote his habilitation thesis on the Thomson model of the atom. A chance meeting with Fritz Haber in Berlin in 1918 led to discussion of how an ionic compound is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Equilibrium
Thermodynamic equilibrium is an axiomatic concept of thermodynamics. It is an internal state of a single thermodynamic system, or a relation between several thermodynamic systems connected by more or less permeable or impermeable walls. In thermodynamic equilibrium, there are no net macroscopic flows of matter nor of energy within a system or between systems. In a system that is in its own state of internal thermodynamic equilibrium, no macroscopic change occurs. Systems in mutual thermodynamic equilibrium are simultaneously in mutual thermal, mechanical, chemical, and radiative equilibria. Systems can be in one kind of mutual equilibrium, while not in others. In thermodynamic equilibrium, all kinds of equilibrium hold at once and indefinitely, until disturbed by a thermodynamic operation. In a macroscopic equilibrium, perfectly or almost perfectly balanced microscopic exchanges occur; this is the physical explanation of the notion of macroscopic equilibrium. A thermodynamic sys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Emissivity
The emissivity of the surface of a material is its effectiveness in emitting energy as thermal radiation. Thermal radiation is electromagnetic radiation that most commonly includes both visible radiation (light) and infrared radiation, which is not visible to human eyes. A portion of the thermal radiation from very hot objects (see photograph) is easily visible to the eye. The emissivity of a surface depends on its chemical composition and geometrical structure. Quantitatively, it is the ratio of the thermal radiation from a surface to the radiation from an ideal black surface at the same temperature as given by the Stefan–Boltzmann law. The ratio varies from 0 to 1. The surface of a perfect black body (with an emissivity of 1) emits thermal radiation at the rate of approximately 448 watts per square metre at room temperature (, ). All real objects have emissivities less than 1.0, and emit radiation at correspondingly lower rates. Emissivities are important in several conte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kirchhoff's Law Of Thermal Radiation
In heat transfer, Kirchhoff's law of thermal radiation refers to wavelength-specific radiative emission and absorption by a material body in thermodynamic equilibrium, including radiative exchange equilibrium. It is a special case of Onsager reciprocal relations as a consequence of the time reversibility of microscopic dynamics, also known as microscopic reversibility. A body at temperature radiates electromagnetic energy. A perfect black body in thermodynamic equilibrium absorbs all light that strikes it, and radiates energy according to a unique law of radiative emissive power for temperature (Stefan–Boltzmann law), universal for all perfect black bodies. Kirchhoff's law states that: Here, the dimensionless coefficient of absorption (or the absorptivity) is the fraction of incident light (power) that is absorbed by the body when it is radiating and absorbing in thermodynamic equilibrium. In slightly different terms, the emissive power of an arbitrary opaque body ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calorimetry
In chemistry and thermodynamics, calorimetry () is the science or act of measuring changes in ''state variables'' of a body for the purpose of deriving the heat transfer associated with changes of its state due, for example, to chemical reactions, physical changes, or phase transitions under specified constraints. Calorimetry is performed with a calorimeter. Scottish physician and scientist Joseph Black, who was the first to recognize the distinction between heat and temperature, is said to be the founder of the science of calorimetry. Indirect calorimetry calculates heat that living organisms produce by measuring either their production of carbon dioxide and nitrogen waste (frequently ammonia in aquatic organisms, or urea in terrestrial ones), or from their consumption of oxygen. Lavoisier noted in 1780 that heat production can be predicted from oxygen consumption this way, using multiple regression. The dynamic energy budget theory explains why this procedure is correct. He ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic System
A thermodynamic system is a body of matter and/or radiation, confined in space by walls, with defined permeabilities, which separate it from its surroundings. The surroundings may include other thermodynamic systems, or physical systems that are not thermodynamic systems. A wall of a thermodynamic system may be purely notional, when it is described as being 'permeable' to all matter, all radiation, and all forces. A state of a thermodynamic system can be fully described in several different ways, by several different sets of thermodynamic state variables. A widely used distinction is between ''isolated'', ''closed'', and ''open'' thermodynamic systems. An isolated thermodynamic system has walls that are non-conductive of heat and perfectly reflective of all radiation, that are rigid and immovable, and that are impermeable to all forms of matter and all forces. (Some writers use the word 'closed' when here the word 'isolated' is being used.) A closed thermodynamic system is c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |