|
Dialysis (chemistry)
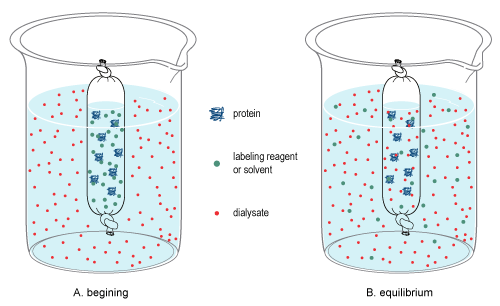
In chemistry, dialysis is the process of separating molecules in solution by the difference in their rates of diffusion through a semipermeable membrane, such as dialysis tubing. Dialysis is a common laboratory technique that operates on the same principle as medical dialysis. In the context of life science research, the most common application of dialysis is for the removal of unwanted small molecules such as salts, reducing agents, or dyes from larger macromolecules such as proteins, DNA, or polysaccharides. Dialysis is also commonly used for buffer exchange and drug binding studies. The concept of dialysis was introduced in 1861 by the Scottish chemist Thomas Graham. He used this technique to separate sucrose (small molecule) and gum Arabic solutes (large molecule) in aqueous solution. He called the diffusible solutes crystalloids and those that would not pass the membrane colloids. From this concept dialysis can be defined as a spontaneous separation process of suspended ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dialysis Figure
Dialysis may refer to: *Dialysis (chemistry), a process of separating molecules in solution **Electrodialysis, used to transport salt ions from one solution to another through an ion-exchange membrane under the influence of an applied electric potential. *Kidney dialysis is the process of removing water, solutes and toxins from the blood of individuals with compromised kidney function, primary types of which are: ** Hemodialysis ** Peritoneal dialysis ** Hemofiltration *Liver dialysis, a detoxification treatment for liver failure. *Dialysis (fly), ''Dialysis'' (fly), a genus of insects in the family Xylophagidae {{Disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anion Exchange Membrane
An anion exchange membrane (AEM) is a semipermeable membrane generally made from ionomers and designed to conduct anions but reject gases such as oxygen or hydrogen. Applications Anion exchange membranes are used in electrolytic cells and fuel cells to separate reactants present around the two electrodes while transporting the anions essential for the cell operation. An important example is the hydroxide anion exchange membrane used to separate the electrodes of a direct methanol fuel cell (DMFC) or direct-ethanol fuel cell (DEFC). Poly(fluorenyl-co-aryl piperidinium) (PFAP)-based anion exchange materials (electrolyte membrane and electrode binder) with high ion conductivity and durability under alkaline conditions has been demonstrated for use to extract hydrogen from water. Performance was 7.68 A/ cm2 at 2 V, some 6x the performance of existing materials. Its yield is about 1.2 times that of commercial proton-exchange membrane technology (6 A/cm2), and it d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Viscose Process
Rayon, also called viscose and commercialised in some countries as sabra silk or cactus silk, is a semi-synthetic fiber made from natural sources of regenerated cellulose, such as wood and related agricultural products. It has the same molecular structure as cellulose. Many types and grades of viscose fibers and films exist. Some imitate the feel and texture of natural fibers such as silk, wool, cotton, and linen. The types that resemble silk are often called artificial silk. It can be woven or knit to make textiles for clothing and other purposes. Rayon production involves solubilizing cellulose to allow turning the fibers into required form. Three common solubilization methods are: * The cuprammonium process (not in use today), using ammoniacal solutions of copper salts * The viscose process, the most common today, using alkali and carbon disulfide * The Lyocell process, using amine oxide, avoids producing neurotoxic carbon disulfide but is more expensive History French ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemicellulose
A hemicellulose (also known as polyose) is one of a number of heteropolymers (matrix polysaccharides), such as arabinoxylans, present along with cellulose in almost all embryophyte, terrestrial plant cell walls. Cellulose is crystalline, strong, and resistant to hydrolysis. Hemicelluloses are branched, shorter in length than cellulose, and also show a propensity to crystallize. They can be hydrolyzed by dilute acid or Base (chemistry), base as well as a myriad of Cellulase, hemicellulase enzymes. Composition Diverse kinds of hemicelluloses are known. Important examples include xylan, glucuronoxylan, arabinoxylan, glucomannan, and xyloglucan. Hemicelluloses are polysaccharides often associated with cellulose, but with distinct compositions and structures. Whereas cellulose is derived exclusively from glucose, hemicelluloses are composed of diverse sugars, and can include the five-carbon sugars xylose and arabinose, the six-carbon sugars glucose, mannose and galactose, and the si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dialysis Tubing
Dialysis tubing, also known as ''Visking tubing'', is an artificial semi-permeable membrane tubing90% of a protein having a molecular mass of at least 10 kDa. Pore sizes typically range from ~10–100 Angstroms for 1K to 50K MWCO membranes. It is important to note that the MWCO of a membrane is not a sharply defined value. Molecules with mass near the MWCO of the membrane will diffuse across the membrane slower than molecules significantly smaller than the MWCO. In order for a molecule to rapidly diffuse across a membrane, it typically needs to be at least 20–50 times smaller than the membranes MWCO rating. Therefore, it is not practical to try separating a 30kDa protein from a 10kDa protein using dialysis across a 20K rated dialysis membrane. Dialysis tubing for laboratory use is typically made of a film of regenerated cellulose or cellulose ester. However; dialysis membranes made of polysulfone, polyethersulfone (PES), etched polycarbonate, or collagen are also extensively us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Weight Cut-off
In ultrafiltration, the molecular weight cut-off or MWCO of a membrane refers to the lowest molecular weight of the solute (in daltons) for which 90% of the solute is retained by (prevented from passing through) the membrane, or the molecular weight of the molecule (e.g. globular protein) that is 90% retained by the membrane. Details This definition is not however standardized, and MWCOs can also be defined as the molecular weight at which 80% of the analytes (or solutes) are prohibited from membrane diffusion. Commercially available microdialysis probes typically have molecular weight cutoffs that range from 1,000 to 300,000 Da, and larger thresholds of filtration are measured in μm. Microdialysis may also be used to separate nanoparticle A nanoparticle or ultrafine particle is a particle of matter 1 to 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 100 nm in only two d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Buffer Solution
A buffer solution is a solution where the pH does not change significantly on dilution or if an acid or base is added at constant temperature. Its pH changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. In nature, there are many living systems that use buffering for pH regulation. For example, the bicarbonate buffering system is used to regulate the pH of blood, and bicarbonate also acts as a buffer in the ocean. Principles of buffering Buffer solutions resist pH change because of a chemical equilibrium between the weak acid HA and its conjugate base A−: When some strong acid is added to an equilibrium mixture of the weak acid and its conjugate base, hydrogen ions (H+) are added, and the equilibrium is shifted to the left, in accordance with Le Chatelier's principle. Because of this, the hydrogen ion concentration increas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Weight Cut-off
In ultrafiltration, the molecular weight cut-off or MWCO of a membrane refers to the lowest molecular weight of the solute (in daltons) for which 90% of the solute is retained by (prevented from passing through) the membrane, or the molecular weight of the molecule (e.g. globular protein) that is 90% retained by the membrane. Details This definition is not however standardized, and MWCOs can also be defined as the molecular weight at which 80% of the analytes (or solutes) are prohibited from membrane diffusion. Commercially available microdialysis probes typically have molecular weight cutoffs that range from 1,000 to 300,000 Da, and larger thresholds of filtration are measured in μm. Microdialysis may also be used to separate nanoparticle A nanoparticle or ultrafine particle is a particle of matter 1 to 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 100 nm in only two d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses Direct current, direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of chemical element, elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity." Etymology The word "electrolysis" was introduced by Michael Faraday in 1834, using the Greek language, Greek words "amber", which since the 17th century was associated with electrical phenomena, and ' meaning "dissolution". Nevertheless, electrolysis, as a tool to study chemical reactions and obtain pure chemical element, elements, precedes the coinage of the term and formal description by Faraday. History In the early nineteenth century, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
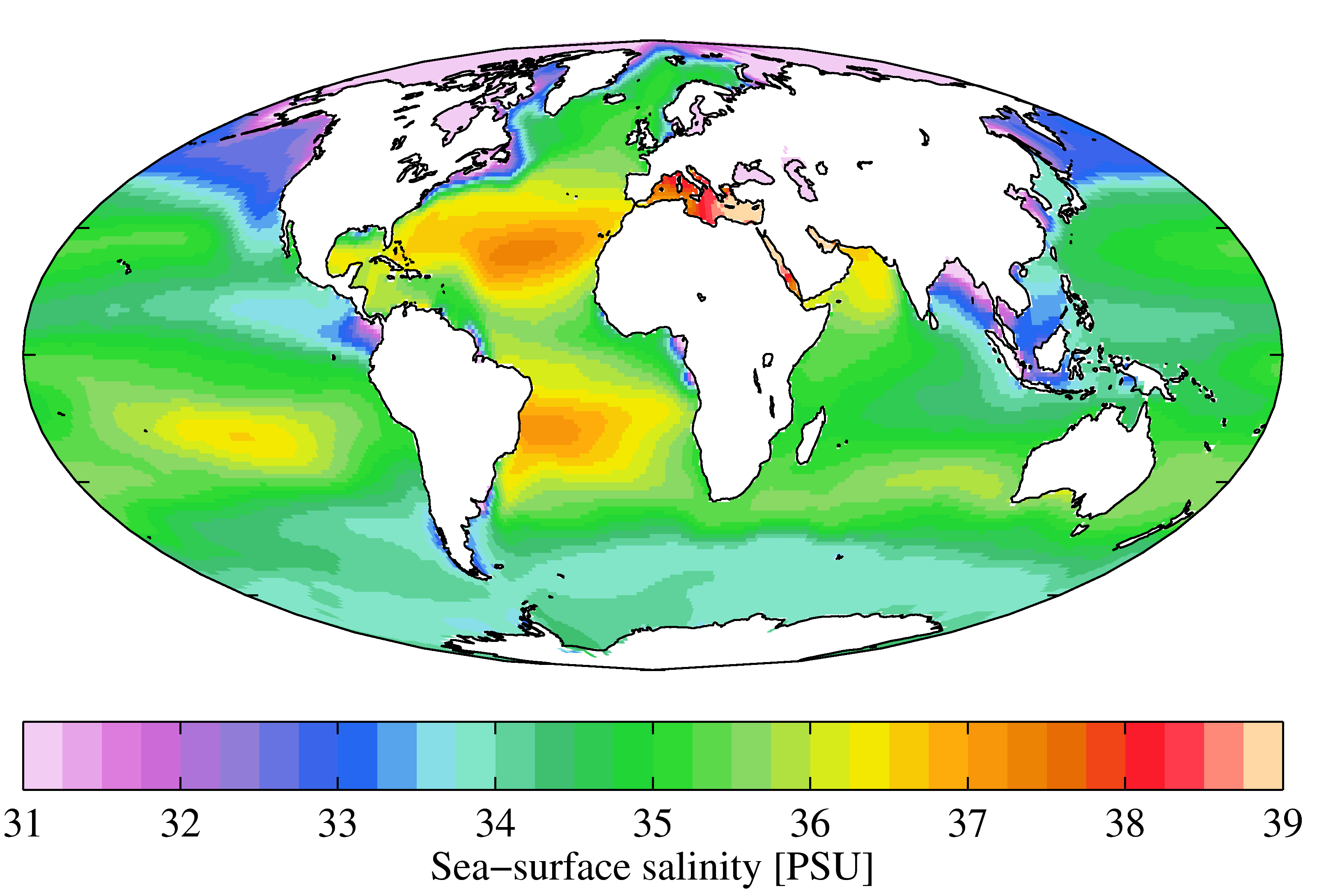
Salinities
Salinity () is the saltiness or amount of salt dissolved in a body of water, called saline water (see also soil salinity). It is usually measured in g/L or g/kg (grams of salt per liter/kilogram of water; the latter is dimensionless and equal to ‰). Salinity is an important factor in determining many aspects of the chemistry of natural waters and of biological processes within it, and is a thermodynamic state variable that, along with temperature and pressure, governs physical characteristics like the density and heat capacity of the water. A contour line of constant salinity is called an ''isohaline'', or sometimes ''isohale''. Definitions Salinity in rivers, lakes, and the ocean is conceptually simple, but technically challenging to define and measure precisely. Conceptually the salinity is the quantity of dissolved salt content of the water. Salts are compounds like sodium chloride, magnesium sulfate, potassium nitrate, and sodium bicarbonate which dissolve into ions. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an electron (the proton-to-electron mass ratio). Protons and neutrons, each with a mass of approximately one Dalton (unit), dalton, are jointly referred to as ''nucleons'' (particles present in atomic nuclei). One or more protons are present in the Atomic nucleus, nucleus of every atom. They provide the attractive electrostatic central force which binds the atomic electrons. The number of protons in the nucleus is the defining property of an element, and is referred to as the atomic number (represented by the symbol ''Z''). Since each chemical element, element is identified by the number of protons in its nucleus, each element has its own atomic number, which determines the number of atomic electrons and consequently the chemical characteristi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |