|
Derjaguin Approximation
The Derjaguin approximation (or sometimes also referred to as the proximity approximation), named after the Russian scientist Boris Derjaguin, expresses the force profile acting between finite size bodies in terms of the force profile between two planar semi-infinite walls. This approximation is widely used to estimate forces between colloidal particles, as forces between two planar bodies are often much easier to calculate. The Derjaguin approximation expresses the force ''F''(''h'') between two bodies as a function of the surface separation as : F(h) = 2 \pi R_ W(h), where ''W''(''h'') is the interaction energy per unit area between the two planar walls and ''R''eff the effective radius. When the two bodies are two spheres of radii ''R''1 and ''R''2, respectively, the effective radius is given by : R_^ = R_1^+R_2^. Experimental force profiles between macroscopic bodies as measured with the surface forces apparatus (SFA) or colloidal probe technique are often reported as the r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
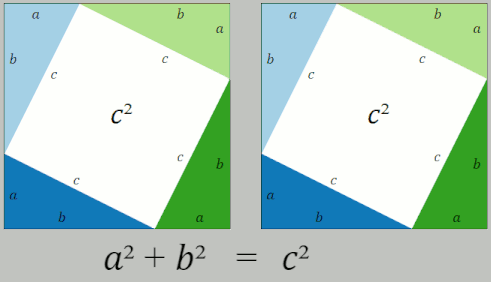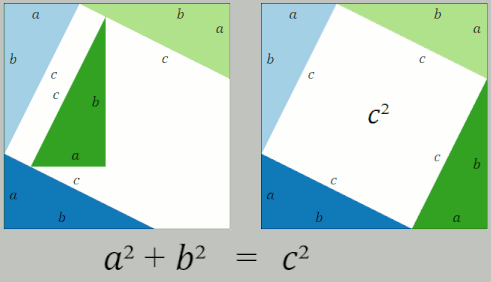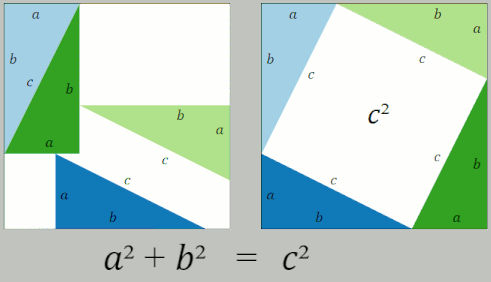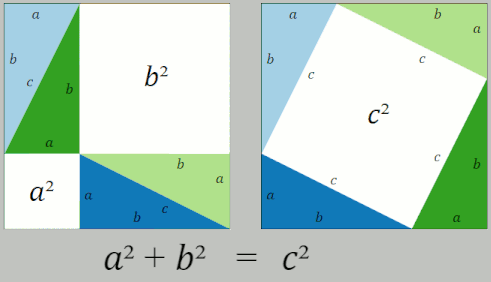
Pythagorean Theorem
In mathematics, the Pythagorean theorem or Pythagoras' theorem is a fundamental relation in Euclidean geometry between the three sides of a right triangle. It states that the area of the square whose side is the hypotenuse (the side opposite the right angle) is equal to the sum of the areas of the squares on the other two sides. This theorem can be written as an equation relating the lengths of the sides ''a'', ''b'' and the hypotenuse ''c'', often called the Pythagorean equation: :a^2 + b^2 = c^2 , The theorem is named for the Greek philosopher Pythagoras, born around 570 BC. The theorem has been proven numerous times by many different methods – possibly the most for any mathematical theorem. The proofs are diverse, including both geometric proofs and algebraic proofs, with some dating back thousands of years. When Euclidean space is represented by a Cartesian coordinate system in analytic geometry, Euclidean distance satisfies the Pythagorean relation: the squared ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Van Der Waals Force
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and therefore more susceptible to disturbance. The van der Waals force quickly vanishes at longer distances between interacting molecules. Named after Dutch physicist Johannes Diderik van der Waals, the van der Waals force plays a fundamental role in fields as diverse as supramolecular chemistry, structural biology, polymer science, nanotechnology, surface science, and condensed matter physics. It also underlies many properties of organic compounds and molecular solids, including their solubility in polar and non-polar media. If no other force is present, the distance between atoms at which the force becomes repulsive rather than attractive as the atoms approach one another is called the van der Waals contact distance; this phenomen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DLVO Theory
The DLVO theory (named after Boris Derjaguin and Lev Landau, Evert Verwey and Theodoor Overbeek) explains the aggregation of aqueous dispersions quantitatively and describes the force between charged surfaces interacting through a liquid medium. It combines the effects of the van der Waals attraction and the electrostatic repulsion due to the so-called double layer of counterions. The electrostatic part of the DLVO interaction is computed in the mean field approximation in the limit of low surface potentials - that is when the potential energy of an elementary charge on the surface is much smaller than the thermal energy scale, k_ T. For two spheres of radius a each having a charge Z (expressed in units of the elementary charge) separated by a center-to-center distance r in a fluid of dielectric constant \epsilon_r containing a concentration n of monovalent ions, the electrostatic potential takes the form of a screened-Coulomb or Yukawa potential, : \beta U(r) = Z^2 \lambda_ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Layer Forces
Double layer forces occur between charged objects across liquids, typically water. This force acts over distances that are comparable to the Debye length, which is on the order of one to a few tenths of nanometers. The strength of these forces increases with the magnitude of the surface charge density (or the electrical surface potential). For two similarly charged objects, this force is repulsive and decays exponentially at larger distances, see figure. For unequally charged objects and eventually at shorted distances, these forces may also be attractive. The theory due to Derjaguin, Landau, Verwey, and Overbeek (DLVO) combines such double layer forces together with Van der Waals forces in order to estimate the actual interaction potential between colloidal particles.W. B. Russel, D. A. Saville, W. R. Schowalter, Colloidal Dispersions. Cambridge University Press: Cambridge, 1989. An electrical double layer develops near charged surfaces (or another charged objects) in aqueous s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Force Microscopy
Atomic force microscopy (AFM) or scanning force microscopy (SFM) is a very-high-resolution type of scanning probe microscopy (SPM), with demonstrated resolution on the order of fractions of a nanometer, more than 1000 times better than the optical diffraction limit. Overview Atomic force microscopy (AFM) is a type of scanning probe microscopy (SPM), with demonstrated resolution on the order of fractions of a nanometer, more than 1000 times better than the optical diffraction limit. The information is gathered by "feeling" or "touching" the surface with a mechanical probe. Piezoelectric elements that facilitate tiny but accurate and precise movements on (electronic) command enable precise scanning. Despite the name, the Atomic Force Microscope does not use the Nuclear force. Abilities The AFM has three major abilities: force measurement, topographic imaging, and manipulation. In force measurement, AFMs can be used to measure the forces between the probe and the sample as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Torque
In physics and mechanics, torque is the rotational equivalent of linear force. It is also referred to as the moment of force (also abbreviated to moment). It represents the capability of a force to produce change in the rotational motion of the body. The concept originated with the studies by Archimedes of the usage of levers, which is reflected in his famous quote: "''Give me a lever and a place to stand and I will move the Earth''". Just as a linear force is a push or a pull, a torque can be thought of as a twist to an object around a specific axis. Torque is defined as the product of the magnitude of the perpendicular component of the force and the distance of the line of action of a force from the point around which it is being determined. The law of conservation of energy can also be used to understand torque. The symbol for torque is typically \boldsymbol\tau, the lowercase Greek letter '' tau''. When being referred to as moment of force, it is commonly denoted by . ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Principal Curvature
In differential geometry, the two principal curvatures at a given point of a surface are the maximum and minimum values of the curvature as expressed by the eigenvalues of the shape operator at that point. They measure how the surface bends by different amounts in different directions at that point. Discussion At each point ''p'' of a differentiable surface in 3-dimensional Euclidean space one may choose a unit ''normal vector''. A '' normal plane'' at ''p'' is one that contains the normal vector, and will therefore also contain a unique direction tangent to the surface and cut the surface in a plane curve, called normal section. This curve will in general have different curvatures for different normal planes at ''p''. The principal curvatures at ''p'', denoted ''k''1 and ''k''2, are the maximum and minimum values of this curvature. Here the curvature of a curve is by definition the reciprocal of the radius of the osculating circle. The curvature is taken to be positive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Derjaguin Approximation Scheme 2
Derjaguin may refer to: *Boris Derjaguin Boris Vladimirovich Derjaguin (or Deryagin; russian: Бори́с Влади́мирович Деря́гин) (9 August 1902 in Moscow – 16 May 1994) was a Soviet and Russian chemist. As a member of the Russian Academy of Sciences, he laid the f ... (1902–1994), Russian chemist ** Derjaguin approximation, expression of force profile interaction between finite size bodies ** DLVO theory, force between charged surfaces interacting through a liquid medium **DMT model of elastic contact; see Contact mechanics {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boris Derjaguin
Boris Vladimirovich Derjaguin (or Deryagin; russian: Бори́с Влади́мирович Деря́гин) (9 August 1902 in Moscow – 16 May 1994) was a Soviet and Russian chemist. As a member of the Russian Academy of Sciences, he laid the foundation of the modern science of colloid A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend ...s and surfaces. An epoch in the development of the physical chemistry of colloids and surfaces is associated with his name. Derjaguin became famous in scientific circles for his work on the stability of colloids and thin films of liquids which is now known as the DLVO theory, after the initials of its authors: Derjaguin, Landau, Verwey, and Overbeek. It is universally included in text books on colloid chemistry and is still widely applied in m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surface Roughness
Surface roughness, often shortened to roughness, is a component of surface finish (surface texture). It is quantified by the deviations in the direction of the normal vector of a real surface from its ideal form. If these deviations are large, the surface is rough; if they are small, the surface is smooth. In surface metrology, roughness is typically considered to be the high-frequency, short-wavelength component of a measured surface. However, in practice it is often necessary to know both the amplitude and frequency to ensure that a surface is fit for a purpose. Roughness plays an important role in determining how a real object will interact with its environment. In tribology, rough surfaces usually wear more quickly and have higher friction coefficients than smooth surfaces. Roughness is often a good predictor of the performance of a mechanical component, since irregularities on the surface may form nucleation sites for cracks or corrosion. On the other hand, roughness ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |