|
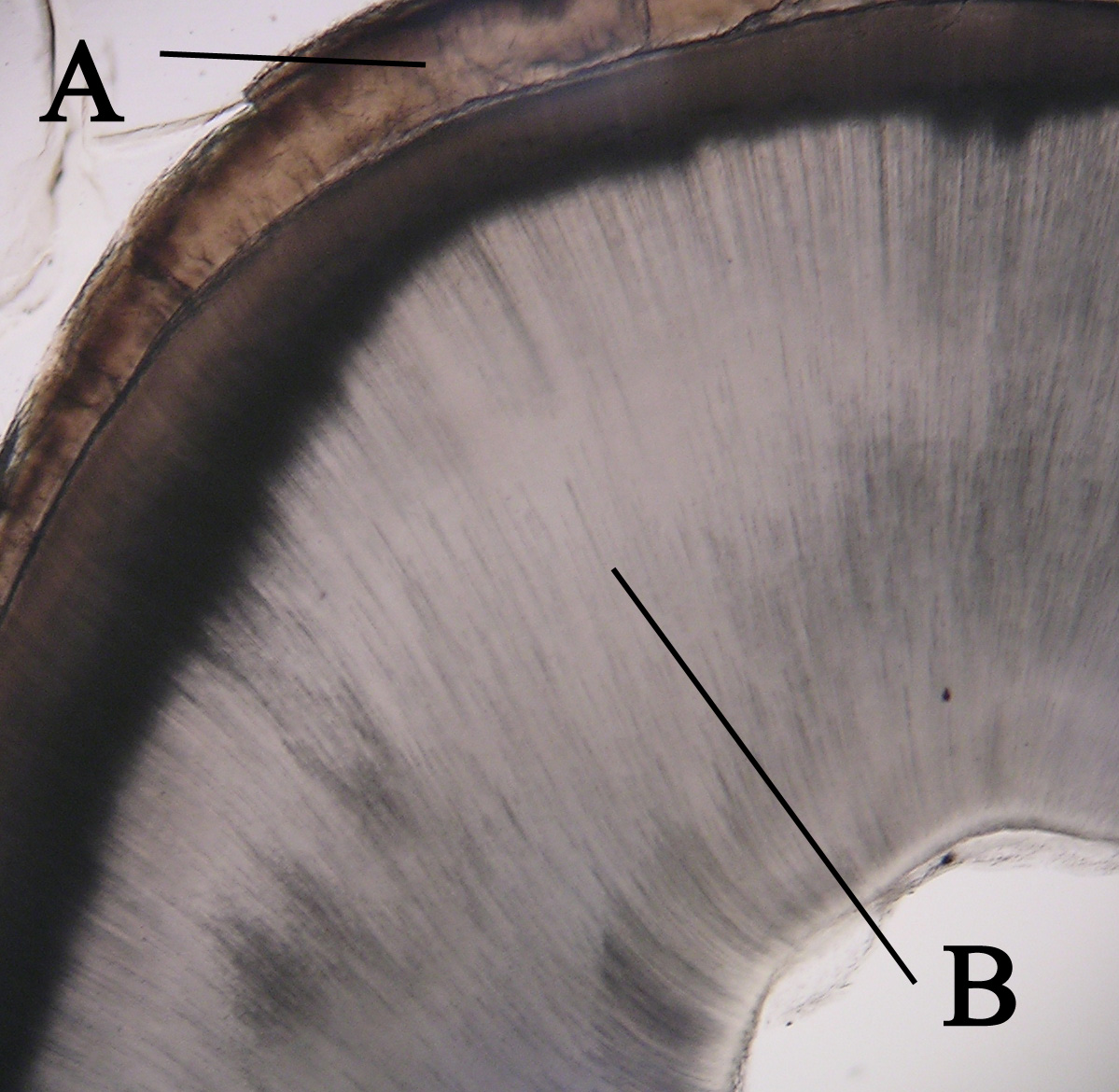
Dentin Appearance
Dentin () (American English) or dentine ( or ) (British English) ( la, substantia eburnea) is a calcified tissue of the body and, along with enamel, cementum, and pulp, is one of the four major components of teeth. It is usually covered by enamel on the crown and cementum on the root and surrounds the entire pulp. By volume, 45% of dentin consists of the mineral hydroxyapatite, 33% is organic material, and 22% is water. Yellow in appearance, it greatly affects the color of a tooth due to the translucency of enamel. Dentin, which is less mineralized and less brittle than enamel, is necessary for the support of enamel. Dentin rates approximately 3 on the Mohs scale of mineral hardness. There are two main characteristics which distinguish dentin from enamel: firstly, dentin forms throughout life; secondly, dentin is sensitive and can become hypersensitive to changes in temperature due to the sensory function of odontoblasts, especially when enamel recedes and dentin channels becom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dentinoenamel Junction
The dentinoenamel junction or dentin-enamel junction (DEJ) is the boundary between the enamel and the underlying dentin that form the solid architecture of a tooth. It is also known as the amelo- dentinal junction, or ADJ. The dentinoenamel junction is thought to be of a scalloped structure which has occurred as an exaptation of the epithelial folding that is undergone during ontogeny. This scalloped exaptation has then provided stress relief during mastication and a reduction in dentin-enamel sliding and has thus, not been selected against, making it an accidental adaptation.T. Pievani & E. Serelli (2011) 'Exaptation in human evolution: how to test adaptive vs exaptive evolutionary hypotheses'. Journal of Anthropological Sciences The ''Journal of Anthropological Sciences'' is an annual peer-reviewed open-access scientific journal covering anthropology. It was established in 1893 as the ''Atti della Società Romana di Antropologia'', and was renamed the ''Rivista di Antropol ... ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tenascin
Tenascins are extracellular matrix glycoproteins. They are abundant in the extracellular matrix of developing vertebrate embryos and they reappear around healing wounds and in the stroma of some tumors. Types There are four members of the tenascin gene family: tenascin-C, tenascin-R, tenascin-X and tenascin-W. * Tenascin-C is the founding member of the gene family. In the embryo it is made by migrating cells like the neural crest; it is also abundant in developing tendons, bone and cartilage. * Tenascin-R is found in the developing and adult nervous system. * Tenascin-X is found primarily in loose connective tissue; mutations in the human tenascin-X gene can lead to a form of Ehlers-Danlos syndrome. * Tenascin-W is found in the kidney and in developing bone. The basic structure is 14 EGF-like repeats towards the N-terminal end, and 8 or more fibronectin-III domains which vary upon species and variant. Tenascin-C is the most intensely studied member of the family. It has an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transferrin
Transferrins are glycoproteins found in vertebrates which bind to and consequently mediate the transport of iron (Fe) through blood plasma. They are produced in the liver and contain binding sites for two Fe3+ ions. Human transferrin is encoded by the ''TF'' gene and produced as a 76 kDa glycoprotein. Transferrin glycoproteins bind iron tightly, but reversibly. Although iron bound to transferrin is less than 0.1% (4 mg) of total body iron, it forms the most vital iron pool with the highest rate of turnover (25 mg/24 h). Transferrin has a molecular weight of around 80 kDa and contains two specific high-affinity Fe(III) binding sites. The affinity of transferrin for Fe(III) is extremely high (association constant is 1020 M−1 at pH 7.4) but decreases progressively with decreasing pH below neutrality. Transferrins are not limited to only binding to iron but also to different metal ions. These glycoproteins are located in various bodily fluids of vertebrates. Some inv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Albumin
Albumin is a family of globular proteins, the most common of which are the serum albumins. All the proteins of the albumin family are water-soluble, moderately soluble in concentrated salt solutions, and experience heat denaturation. Albumins are commonly found in blood plasma and differ from other blood proteins in that they are not glycosylated. Substances containing albumins are called ''albuminoids''. A number of blood transport proteins are evolutionarily related in the albumin family, including serum albumin, alpha-fetoprotein, vitamin D-binding protein and afamin. This family is only found in vertebrates. ''Albumins'' in a less strict sense can mean other proteins that coagulate under certain conditions. See for lactalbumin, ovalbumin and plant "2S albumin". Function Albumins in general are transport proteins that bind to various ligands and carry them around. Human types include: * Human serum albumin is the main protein of human blood plasma. It makes up around 50 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Odontoblast Process
An odontoblast process (also called Tomes's fibers or Tomes fibers, or by a dated term Tomes's fibrils) is an extension of a cell called an odontoblast, which forms dentin in a tooth. The odontoblast process is located in dentinal tubules. It forms during dentinogenesis and results from a part of the odontoblast staying in its location as the main body of the odontoblast moves toward the center of the tooth's pulp. The odontoblast process causes the secretion of hydroxyapatite crystals and mineralization of the matrix secreted by the odontoblasts. References * See also *Tomes's process Tomes's processes (also called Tomes processes) are a histology, histologic landmark identified on an ameloblast, cells involved in the production of tooth enamel. During the synthesis of enamel, the ameloblast moves away from the Tooth enamel, ena ... * John Tomes Tooth development {{dentistry-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dental-enamel Junction
The dentinoenamel junction or dentin-enamel junction (DEJ) is the boundary between the enamel and the underlying dentin that form the solid architecture of a tooth. It is also known as the amelo- dentinal junction, or ADJ. The dentinoenamel junction is thought to be of a scalloped structure which has occurred as an exaptation of the epithelial folding that is undergone during ontogeny. This scalloped exaptation has then provided stress relief during mastication and a reduction in dentin-enamel sliding and has thus, not been selected against, making it an accidental adaptation.T. Pievani & E. Serelli (2011) 'Exaptation in human evolution: how to test adaptive vs exaptive evolutionary hypotheses'. Journal of Anthropological Sciences The ''Journal of Anthropological Sciences'' is an annual peer-reviewed open-access scientific journal covering anthropology. It was established in 1893 as the ''Atti della Società Romana di Antropologia'', and was renamed the ''Rivista di Antropol ... ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Histology
Histology, also known as microscopic anatomy or microanatomy, is the branch of biology which studies the microscopic anatomy of biological tissues. Histology is the microscopic counterpart to gross anatomy, which looks at larger structures visible without a microscope. Although one may divide microscopic anatomy into ''organology'', the study of organs, ''histology'', the study of tissues, and ''cytology'', the study of cells, modern usage places all of these topics under the field of histology. In medicine, histopathology is the branch of histology that includes the microscopic identification and study of diseased tissue. In the field of paleontology, the term paleohistology refers to the histology of fossil organisms. Biological tissues Animal tissue classification There are four basic types of animal tissues: muscle tissue, nervous tissue, connective tissue, and epithelial tissue. All animal tissues are considered to be subtypes of these four principal tissue types ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Matrix Metalloproteinase
Matrix metalloproteinases (MMPs), also known as matrix metallopeptidases or matrixins, are metalloproteinases that are calcium-dependent zinc-containing endopeptidases; other family members are adamalysins, serralysins, and astacins. The MMPs belong to a larger family of proteases known as the metzincin superfamily. Collectively, these enzymes are capable of degrading all kinds of extracellular matrix proteins, but also can process a number of bioactive molecules. They are known to be involved in the cleavage of cell surface receptors, the release of apoptotic ligands (such as the FAS ligand), and chemokine/cytokine inactivation. MMPs are also thought to play a major role in cell behaviors such as cell proliferation, migration (adhesion/dispersion), differentiation, angiogenesis, apoptosis, and host defense. They were first described in vertebrates (1962), including humans, but have since been found in invertebrates and plants. They are distinguished from other endopeptida ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaline Phosphatase
The enzyme alkaline phosphatase (EC 3.1.3.1, alkaline phosphomonoesterase; phosphomonoesterase; glycerophosphatase; alkaline phosphohydrolase; alkaline phenyl phosphatase; orthophosphoric-monoester phosphohydrolase (alkaline optimum), systematic name phosphate-monoester phosphohydrolase (alkaline optimum)) catalyses the following reaction: : a phosphate monoester + H2O = an alcohol + phosphate Alkaline phosphatase has the physiological role of dephosphorylating compounds. The enzyme is found across a multitude of organisms, prokaryotes and eukaryotes alike, with the same general function but in different structural forms suitable to the environment they function in. Alkaline phosphatase is found in the periplasmic space of '' E. coli'' bacteria. This enzyme is heat stable and has its maximum activity at high pH. In humans, it is found in many forms depending on its origin within the body – it plays an integral role in metabolism within the liver and development withi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteoglycan
Proteoglycans are proteins that are heavily glycosylated. The basic proteoglycan unit consists of a "core protein" with one or more covalently attached glycosaminoglycan (GAG) chain(s). The point of attachment is a serine (Ser) residue to which the glycosaminoglycan is joined through a tetrasaccharide bridge (e.g. chondroitin sulfate- GlcA- Gal-Gal- Xyl-PROTEIN). The Ser residue is generally in the sequence -Ser-Gly-X-Gly- (where X can be any amino acid residue but proline), although not every protein with this sequence has an attached glycosaminoglycan. The chains are long, linear carbohydrate polymers that are negatively charged under physiological conditions due to the occurrence of sulfate and uronic acid groups. Proteoglycans occur in connective tissue. Types Proteoglycans are categorized by their relative size (large and small) and the nature of their glycosaminoglycan chains. Types include: Certain members are considered members of the "small leucine-rich proteoglyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoprotein
A phosphoprotein is a protein that is posttranslationally modified by the attachment of either a single phosphate group, or a complex molecule such as 5'-phospho-DNA, through a phosphate group. The target amino acid is most often serine, threonine, or tyrosine residues (mostly in eukaryotes), or aspartic acid or histidine residues (mostly in prokaryotes). Biological function The phosphorylation of proteins is a major regulatory mechanism in cells. Clinical significance Phosphoproteins have been proposed as biomarkers for breast cancer. See also *Protein phosphorylation Protein phosphorylation is a reversible post-translational modification of proteins in which an amino acid residue is phosphorylated by a protein kinase by the addition of a covalently bound phosphate group. Phosphorylation alters the structural ... References Phosphoproteins {{protein-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |