|
Deacetylate
: In organic chemistry, acetylation is an organic esterification reaction with acetic acid. It introduces an acetyl group into a chemical compound. Such compounds are termed ''acetate esters'' or simply '' acetates''. Deacetylation is the opposite reaction, the removal of an acetyl group from a chemical compound. Organic synthesis Acetate esters and acetamides are generally prepared by acetylations. Acetylations are often used in making C-acetyl bonds in Friedel-Crafts reactions. Carbanions and their equivalents are susceptible to acetylations. Acetylation reagents Many acetylations are achieved using these three reagents: * Acetic anhydride. This reagent is common in the laboratory; its use cogenerates acetic acid. *Acetyl chloride. This reagent is also common in the laboratory, but its use cogenerates hydrogen chloride, which can be undesirable. *Ketene. At one time acetic anhydride was prepared by the reaction of ketene with acetic acid: :H2C=C=O + CH3COOH -> (CH3CO)2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aspirin Synthesis
Aspirin, also known as acetylsalicylic acid (ASA), is a nonsteroidal anti-inflammatory drug (NSAID) used to reduce pain, fever, and/or inflammation, and as an antithrombotic. Specific inflammatory conditions which aspirin is used to treat include Kawasaki disease, pericarditis, and rheumatic fever. Aspirin is also used long-term to help prevent further heart attacks, ischaemic strokes, and blood clots in people at high risk. For pain or fever, effects typically begin within 30 minutes. Aspirin works similarly to other NSAIDs but also suppresses the normal functioning of platelets. One common adverse effect is an upset stomach. More significant side effects include stomach ulcers, stomach bleeding, and worsening asthma. Bleeding risk is greater among those who are older, drink alcohol, take other NSAIDs, or are on other blood thinners. Aspirin is not recommended in the last part of pregnancy. It is not generally recommended in children with infections because of the risk of R ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyol
In organic chemistry, a polyol is an organic compound containing multiple hydroxyl groups (). The term "polyol" can have slightly different meanings depending on whether it is used in food science or polymer chemistry. Polyols containing two, three and four hydroxyl groups are diols, triols, and tetrols, respectively. Classification Polyols may be classified according to their chemistry. Some of these chemistries are polyether, polyester, polycarbonate and also acrylic polyols. Polyether polyols may be further subdivided and classified as polyethylene oxide or polyethylene glycol (PEG), polypropylene glycol (PPG) and Polytetrahydrofuran or PTMEG. These have 2, 3 and 4 carbons respectively per oxygen atom in the repeat unit. Polycaprolactone polyols are also commercially available. There is also an increasing trend to use biobased (and hence renewable) polyols. Uses Polyether polyols have numerous uses. As an example, polyurethane foam is a big user of polyether polyols. Polye ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Esters typically have a pleasant smell; those of low molecular weight are commonly used as fragrances and are found in essential oils and pheromones. They perform as high-grade solvents for a broad array of plastics, plasticizers, resins, and lacquers, and are one of the largest classes of synthetic lubricants on the commercial market. Polyesters are important plastics, with monomers linked by ester moieties. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties. '' Nomenclature Etymology Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, such as in the amino acids asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid () with the hydroxyl group () replaced by an amine group (); or, equivalently, an acyl (alkanoyl) group () joined to an amine group. Common examples of amides are acetamide (), benzamide (), and dimethylformamide (). Amides are qualified as primary, secondary, and tertiary according to whether the amine subgroup has the form , , or , where R and R' are groups other than hydrogen. The core of amides is called the amide group (specifically, carboxamide group). Amides are pervasive in nature and technology. Proteins and important plastics l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acylation
In chemistry, acylation (or alkanoylation) is the chemical reaction in which an acyl group () is added to a compound. The compound providing the acyl group is called the acylating agent. Because they form a strong electrophile when treated with some metal catalysts, acyl halides are commonly used as acylating agents. For example, Friedel–Crafts acylation uses acetyl chloride (ethanoyl chloride or ) as the agent and aluminum chloride () as a catalyst to add an ethanoyl ( acetyl) group to benzene: The mechanism of this reaction is electrophilic aromatic substitution. Acyl halides and acid anhydrides of carboxylic acids are also commonly used acylating agents. In some cases, active esters exhibit comparable reactivity. All react with amines to form amides and with alcohols to form esters by nucleophilic acyl substitution. Acylation can be used to prevent rearrangement reactions that would normally occur in alkylation. To do this an acylation reaction is performed, then the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetoxy Group
In organic chemistry, the acetoxy group (abbr. AcO or OAc; IUPAC name: acetyloxy), is a functional group with the formula and the structure . As the ''-oxy'' suffix implies, it differs from the acetyl group () by the presence of an additional oxygen atom. The name acetoxy is the short form of ''acetyl-oxy''. Functionality An acetoxy group may be used as a protection for an alcohol functionality in a synthetic route although the protecting group itself is called an acetyl group. Alcohol protection There are several options of introducing an acetoxy functionality in a molecule from an alcohol (in effect protecting the alcohol by acetylation): * Acetyl halide, such as acetyl chloride in the presence of a base like triethylamine * Activated ester form of acetic acid, such as a N-hydroxysuccinimide ester, although this is not advisable due to higher costs and difficulties. * Acetic anhydride in the presence of base with a catalyst such as pyridine with a bit of DMAP added. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Histone Deacetylase
Histone deacetylases (, HDAC) are a class of enzymes that remove acetyl groups (O=C-CH3) from an ε-N-acetyl lysine amino acid on a histone, allowing the histones to wrap the DNA more tightly. This is important because DNA is wrapped around histones, and DNA expression is regulated by acetylation and de-acetylation. Its action is opposite to that of histone acetyltransferase. HDAC proteins are now also called lysine deacetylases (KDAC), to describe their function rather than their target, which also includes non-histone proteins. HDAC super family Together with the acetylpolyamine amidohydrolases and the acetoin utilization proteins, the histone deacetylases form an ancient protein superfamily known as the histone deacetylase superfamily. Classes of HDACs in higher eukaryotes HDACs, are classified in four classes depending on sequence homology to the yeast original enzymes and domain organization: HDAC (except class III) contain zinc and are known as Zn2+-dependent hi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Histone Acetyltransferase
Histone acetyltransferases (HATs) are enzymes that acetylate conserved lysine amino acids on histone proteins by transferring an acetyl group from acetyl-CoA to form ε-''N''-acetyllysine. DNA is wrapped around histones, and, by transferring an acetyl group to the histones, genes can be turned on and off. In general, histone acetylation increases gene expression. In general, histone acetylation is linked to transcriptional activation and associated with euchromatin. Euchromatin, which is less densely compact, allows transcription factors to bind more easily to regulatory sites on DNA, causing transcriptional activation. When it was first discovered, it was thought that acetylation of lysine neutralizes the positive charge normally present, thus reducing affinity between histone and (negatively charged) DNA, which renders DNA more accessible to transcription factors. Research has emerged, since, to show that lysine acetylation and other posttranslational modifications of hist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal bleeding, prolonged cough, unexplained weight loss, and a change in bowel movements. While these symptoms may indicate cancer, they can also have other causes. Over 100 types of cancers affect humans. Tobacco use is the cause of about 22% of cancer deaths. Another 10% are due to obesity, poor diet, lack of physical activity or excessive drinking of alcohol. Other factors include certain infections, exposure to ionizing radiation, and environmental pollutants. In the developing world, 15% of cancers are due to infections such as ''Helicobacter pylori'', hepatitis B, hepatitis C, human papillomavirus infection, Epstein–Barr virus and human immunodeficiency virus (HIV). These factors act, at least partly, by changing the genes of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gene Expression
Gene expression is the process by which information from a gene is used in the synthesis of a functional gene product that enables it to produce end products, protein or non-coding RNA, and ultimately affect a phenotype, as the final effect. These products are often proteins, but in non-protein-coding genes such as transfer RNA (tRNA) and small nuclear RNA (snRNA), the product is a functional non-coding RNA. Gene expression is summarized in the central dogma of molecular biology first formulated by Francis Crick in 1958, further developed in his 1970 article, and expanded by the subsequent discoveries of reverse transcription and RNA replication. The process of gene expression is used by all known life—eukaryotes (including multicellular organisms), prokaryotes (bacteria and archaea), and utilized by viruses—to generate the macromolecular machinery for life. In genetics, gene expression is the most fundamental level at which the genotype gives rise to the phenotype, '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
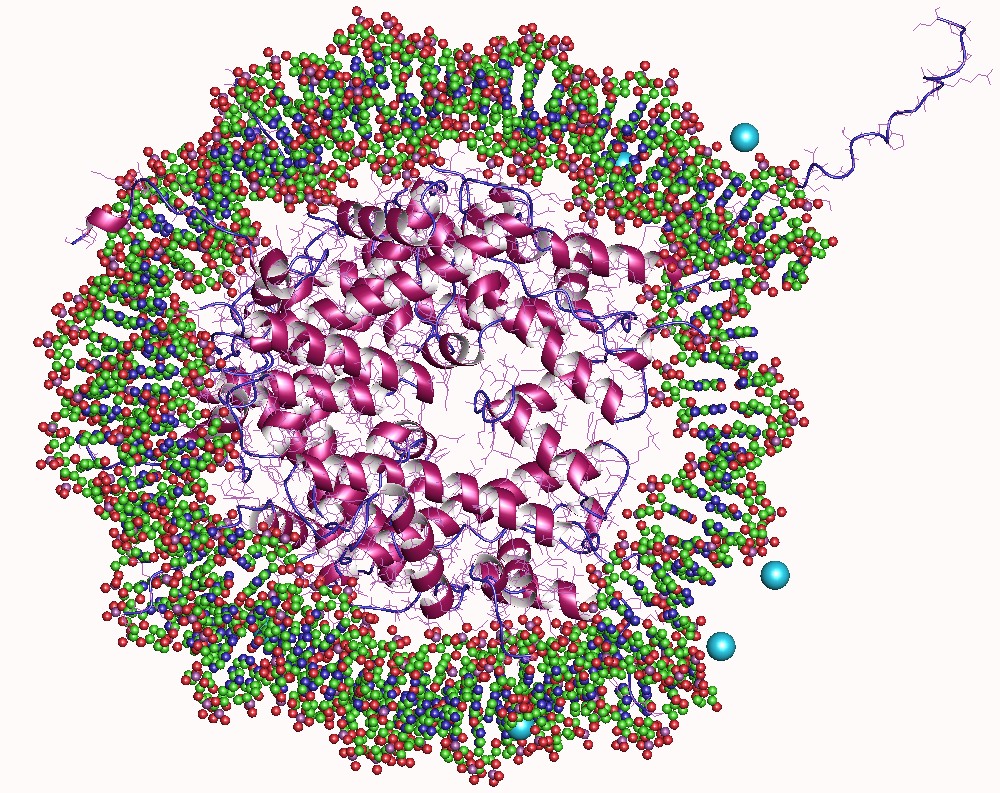
Histone Acetylation And Deacetylation
In biology, histones are highly basic proteins abundant in lysine and arginine residues that are found in eukaryotic cell nuclei. They act as spools around which DNA winds to create structural units called nucleosomes. Nucleosomes in turn are wrapped into 30- nanometer fibers that form tightly packed chromatin. Histones prevent DNA from becoming tangled and protect it from DNA damage. In addition, histones play important roles in gene regulation and DNA replication. Without histones, unwound DNA in chromosomes would be very long. For example, each human cell has about 1.8 meters of DNA if completely stretched out; however, when wound about histones, this length is reduced to about 90 micrometers (0.09 mm) of 30 nm diameter chromatin fibers. There are five families of histones which are designated H1/H5 (linker histones), H2, H3, and H4 (core histones). The nucleosome core is formed of two H2A-H2B dimers and a H3-H4 tetramer. The tight wrapping of DNA around hist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. It contains an α-amino group (which is in the protonated form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain lysyl ((CH2)4NH2), classifying it as a basic, charged (at physiological pH), aliphatic amino acid. It is encoded by the codons AAA and AAG. Like almost all other amino acids, the α-carbon is chiral and lysine may refer to either enantiomer or a racemic mixture of both. For the purpose of this article, lysine will refer to the biologically active enantiomer L-lysine, where the α-carbon is in the ''S'' configuration. The human body cannot synthesize lysine. It is essential in humans and must therefore be obtained from the diet. In organisms that synthesise lysine, two main biosynthetic pathways exist, the diaminopimelate and α-aminoadipate pathways, which employ distinct e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |