|
Dangling Bonds
In chemistry, a dangling bond is an unsatisfied valence on an immobilized atom. An atom with a dangling bond is also referred to as an immobilized free radical or an immobilized radical, a reference to its structural and chemical similarity to a free radical. When speaking of a dangling bond, one is generally referring to the state described above, containing one electron and thus leading to a neutrally charged atom. There are also dangling bond defects containing two or no electrons. These are negatively and positively charged respectively. Dangling bonds with two electrons have an energy close to the valence band of the material and those with none have an energy that is closer to the conduction band. Properties In order to gain enough electrons to fill their valence shells (see also octet rule), many atoms will form covalent bonds with other atoms. In the simplest case, that of a single bond, two atoms each contribute one unpaired electron, and the resulting pair of electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dangling Bonds In Amorphous Silicon (comput ...
Dangle, dangler or dangling may refer to: * Dangler (plot device), an unresolved plot line in a story * Dangle (espionage), an agent of one side who pretends to be interested in defecting to another side * Dangle, a type of earring * Dangle, in ice hockey, a variety of moves where a player dekes (fakes) out a goalie or player (it originally meant to skate fast with the puck) * Dangle, in lacrosse, a complete defeat of a defender or goalie achieved by performing complex stick moves and tricks People with the surname * Lloyd Dangle, American writer and artist * Lt. Jim Dangle, a ''Reno 911!'' character See also * Dangling modifier (or dangling participle), a type of misplaced grammatical phrase * Dangling pointer Dangling pointers and wild pointers in computer programming are pointers that do not point to a valid object of the appropriate type. These are special cases of memory safety violations. More generally, dangling references and wild references ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exponential Decay
A quantity is subject to exponential decay if it decreases at a rate proportional to its current value. Symbolically, this process can be expressed by the following differential equation, where is the quantity and (lambda) is a positive rate called the exponential decay constant, disintegration constant, rate constant, or transformation constant: :\frac = -\lambda N. The solution to this equation (see derivation below) is: :N(t) = N_0 e^, where is the quantity at time , is the initial quantity, that is, the quantity at time . Measuring rates of decay Mean lifetime If the decaying quantity, ''N''(''t''), is the number of discrete elements in a certain set, it is possible to compute the average length of time that an element remains in the set. This is called the mean lifetime (or simply the lifetime), where the exponential time constant, \tau, relates to the decay rate constant, λ, in the following way: :\tau = \frac. The mean lifetime can be looked at as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Germanium Silicide
Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors silicon and tin. Like silicon, germanium naturally reacts and forms complexes with oxygen in nature. Because it seldom appears in high concentration, germanium was discovered comparatively late in the discovery of the elements. Germanium ranks near fiftieth in relative abundance of the elements in the Earth's crust. In 1869, Dmitri Mendeleev predicted its existence and some of its properties from its position on his periodic table, and called the element ekasilicon. In 1886, Clemens Winkler at Freiberg University found the new element, along with silver and sulfur, in the mineral argyrodite. Winkler named the element after his country, Germany. Germanium is mined primarily from sphalerite (the primary ore of zinc), though germanium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
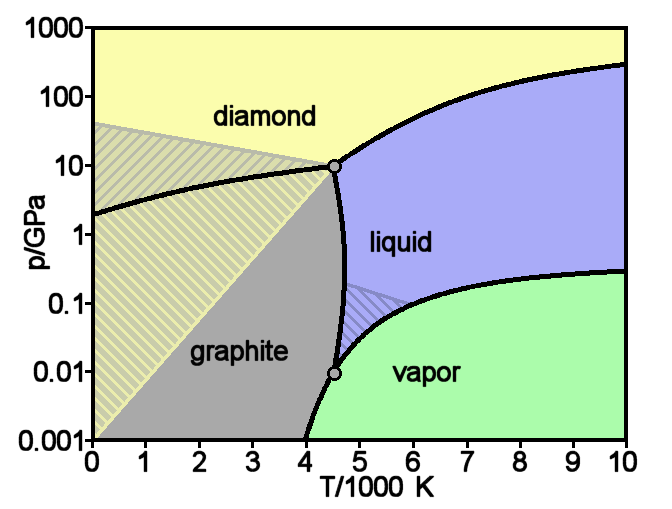
Graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on large scale (300 kton/year, in 1989) for uses in pencils, lubricants, and electrodes. Under high pressures and temperatures it converts to diamond. It is a weak conductor of heat and electricity. Types and varieties Natural graphite The principal types of natural graphite, each occurring in different types of ore deposits, are * Crystalline small flakes of graphite (or flake graphite) occurs as isolated, flat, plate-like particles with hexagonal edges if unbroken. When broken the edges can be irregular or angular; * Amorphous graphite: very fine flake graphite is sometimes called amorphous; * Lump graphite (or vein graphite) occurs in fissure veins or fractures and appears as massive platy intergrowths of fibrous or acicular crystalline ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surface Reorganisation In Silicon Pairs Dangling Bonds
A surface, as the term is most generally used, is the outermost or uppermost layer of a physical object or space. It is the portion or region of the object that can first be perceived by an observer using the senses of sight and touch, and is the portion with which other materials first interact. The surface of an object is more than "a mere geometric solid", but is "filled with, spread over by, or suffused with perceivable qualities such as color and warmth". The concept of surface has been abstracted and formalized in mathematics, specifically in geometry. Depending on the properties on which the emphasis is given, there are several non equivalent such formalizations, that are all called ''surface'', sometimes with some qualifier, such as algebraic surface, smooth surface or fractal surface. The concept of surface and its mathematical abstraction are both widely used in physics, engineering, computer graphics, and many other disciplines, primarily in representing the surfaces ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Hole
In physics, chemistry, and electronic engineering, an electron hole (often simply called a hole) is a quasiparticle which is the lack of an electron at a position where one could exist in an atom or atomic lattice. Since in a normal atom or crystal lattice the negative charge of the electrons is balanced by the positive charge of the atomic nuclei, the absence of an electron leaves a net positive charge at the hole's location. Holes in a metal or semiconductor crystal lattice can move through the lattice as electrons can, and act similarly to positively-charged particles. They play an important role in the operation of semiconductor devices such as transistors, diodes and integrated circuits. If an electron is excited into a higher state it leaves a hole in its old state. This meaning is used in Auger electron spectroscopy (and other x-ray techniques), in computational chemistry, and to explain the low electron-electron scattering-rate in crystals (metals, semiconduct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Staebler–Wronski Effect
The Staebler–Wronski Effect (SWE) refers to light-induced metastability, metastable changes in the properties of hydrogenated amorphous silicon. The defect density of hydrogenated amorphous silicon (a-Si:H) increases with light exposure, causing an increase in the carrier generation and recombination, recombination current and reducing the efficiency of the conversion of sunlight into electricity. It was discovered by David L. Staebler and Christopher R. Wronski in 1977. They showed that the dark current (physics), dark current and photoconductivity of hydrogenated amorphous silicon can be reduced significantly by prolonged illumination with intense light. However, on heating the samples to above 150 °C, they could reverse the effect. Explanation Some experimental results * Photoconductivity and dark conductivity decrease rapidly at first before stabilizing at a lower value. * Interruptions in the illumination has no effect on the subsequent rate of change. Once the samp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoconductivity
Photoconductivity is an optical and electrical phenomenon in which a material becomes more electrically conductive due to the absorption of electromagnetic radiation such as visible light, ultraviolet light, infrared light, or gamma radiation. When light is absorbed by a material such as a semiconductor, the number of free electrons and holes increases, resulting in increased electrical conductivity. To cause excitation, the light that strikes the semiconductor must have enough energy to raise electrons across the band gap, or to excite the impurities within the band gap. When a bias voltage and a load resistor are used in series with the semiconductor, a voltage drop across the load resistors can be measured when the change in electrical conductivity of the material varies the current through the circuit. Classic examples of photoconductive materials include: * photographic film: Kodachrome, Fujifilm, Agfachrome, Ilford, ''etc.'', based on silver sulfide and silver bromide. * th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polarization (waves)
Polarization (also polarisation) is a property applying to transverse waves that specifies the geometrical orientation of the oscillations. In a transverse wave, the direction of the oscillation is perpendicular to the direction of motion of the wave. A simple example of a polarized transverse wave is vibrations traveling along a taut string ''(see image)''; for example, in a musical instrument like a guitar string. Depending on how the string is plucked, the vibrations can be in a vertical direction, horizontal direction, or at any angle perpendicular to the string. In contrast, in longitudinal waves, such as sound waves in a liquid or gas, the displacement of the particles in the oscillation is always in the direction of propagation, so these waves do not exhibit polarization. Transverse waves that exhibit polarization include electromagnetic waves such as light and radio waves, gravitational waves, and transverse sound waves (shear waves) in solids. An electromagnetic wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tight Binding
In solid-state physics, the tight-binding model (or TB model) is an approach to the calculation of electronic band structure using an approximate set of wave functions based upon superposition of wave functions for isolated atoms located at each atomic site. The method is closely related to the LCAO method (linear combination of atomic orbitals method) used in chemistry. Tight-binding models are applied to a wide variety of solids. The model gives good qualitative results in many cases and can be combined with other models that give better results where the tight-binding model fails. Though the tight-binding model is a one-electron model, the model also provides a basis for more advanced calculations like the calculation of surface states and application to various kinds of many-body problem and quasiparticle calculations. Introduction The name "tight binding" of this electronic band structure model suggests that this quantum mechanical model describes the properties of tightly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Franck–Condon Principle
The Franck–Condon principle (named for James Franck and Edward Condon) is a rule in spectroscopy and quantum chemistry that explains the intensity of vibronic transitions (the simultaneous changes in electronic and vibrational energy levels of a molecule due to the absorption or emission of a photon of the appropriate energy). The principle states that during an electronic transition, a change from one vibrational energy level to another will be more likely to happen if the two vibrational wave functions overlap more significantly. Overview The Franck–Condon principle has a well-established semiclassical interpretation based on the original contributions of James Franck. Electronic transitions are relatively instantaneous compared with the time scale of nuclear motions, therefore if the molecule is to move to a new vibrational level during the electronic transition, this new vibrational level must be instantaneously compatible with the nuclear positions and momenta of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |