|
Cystine
Cystine is the oxidized derivative of the amino acid cysteine and has the formula (SCH2CH(NH2)CO2H)2. It is a white solid that is poorly soluble in water. As a residue in proteins, cystine serves two functions: a site of redox reactions and a mechanical linkage that allows proteins to retain their three-dimensional structure. Formation and reactions Structure Cystine is the disulfide derived from the amino acid cysteine. The conversion can be viewed as an oxidation: : Cystine contains a disulfide bond, two amine groups, and two carboxylic acid groups. As for other amino acids, the amine and carboxylic acid groups exist is rapid equilibrium with the ammonium-carboxylate tautomer. The great majority of the literature concerns the ''l,l-''cystine, derived from ''l''-cysteine. Other isomers include ''d,d''-cystine and the meso isomer d,l-cystine, neither of which is biologically significant. Occurrence Cystine is common in many foods such as eggs, meat, dairy products, and whole ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cystinuria
Cystinuria is an inherited autosomal recessive disease characterized by high concentrations of the amino acid cystine in the urine, leading to the formation of cystine stones in the kidneys, ureters, and bladder. It is a type of aminoaciduria. "Cystine", not "cysteine," is implicated in this disease; the former is a dimer of the latter. Presentation Cystinuria is a cause of recurrent kidney stones. It is a disease involving the defective transepithelial transport of cystine and dibasic amino acids in the kidney and intestine, and is one of many causes of kidney stones. If not treated properly, the disease could cause serious damage to the kidneys and surrounding organs, and in some rare cases death. The stones may be identified by a positive nitroprusside cyanide test. The crystals are usually hexagonal, translucent, white. Upon removal, the stones may be pink or yellow in color, but later they turn to greenish due to exposure to air. Cystinuria is usually asymptomatic whe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SLC7A11
Cystine/glutamate transporter is an antiporter that in humans is encoded by the ''SLC7A11'' gene. The SLC7A11 gene codes for a sodium-independent cystine- glutamate antiporter that is chloride dependent, known as system Xc- or xCT. It regulates synaptic activity by stimulating extrasynaptic receptors and performs nonvesicular glutamate release. This gene is highly expressed by astrocytes and couples the uptake of one molecule of cystine with the release of one molecule of glutamate. The dimer cystine gets taken up by glial cells and the monomer of cystine, cysteine, is taken up by neurons. The expression of Xc- was detected throughout the brain with higher expression found in the basolateral amygdala, the Retina and the prefrontal cortex. The inhibition of system Xc- has been found to alter a number of behaviors, which suggests that it plays a key role in excitatory signaling. Structure SLC7A11 is a member of a heterodimeric Na+-independent anionic amino acid transport ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
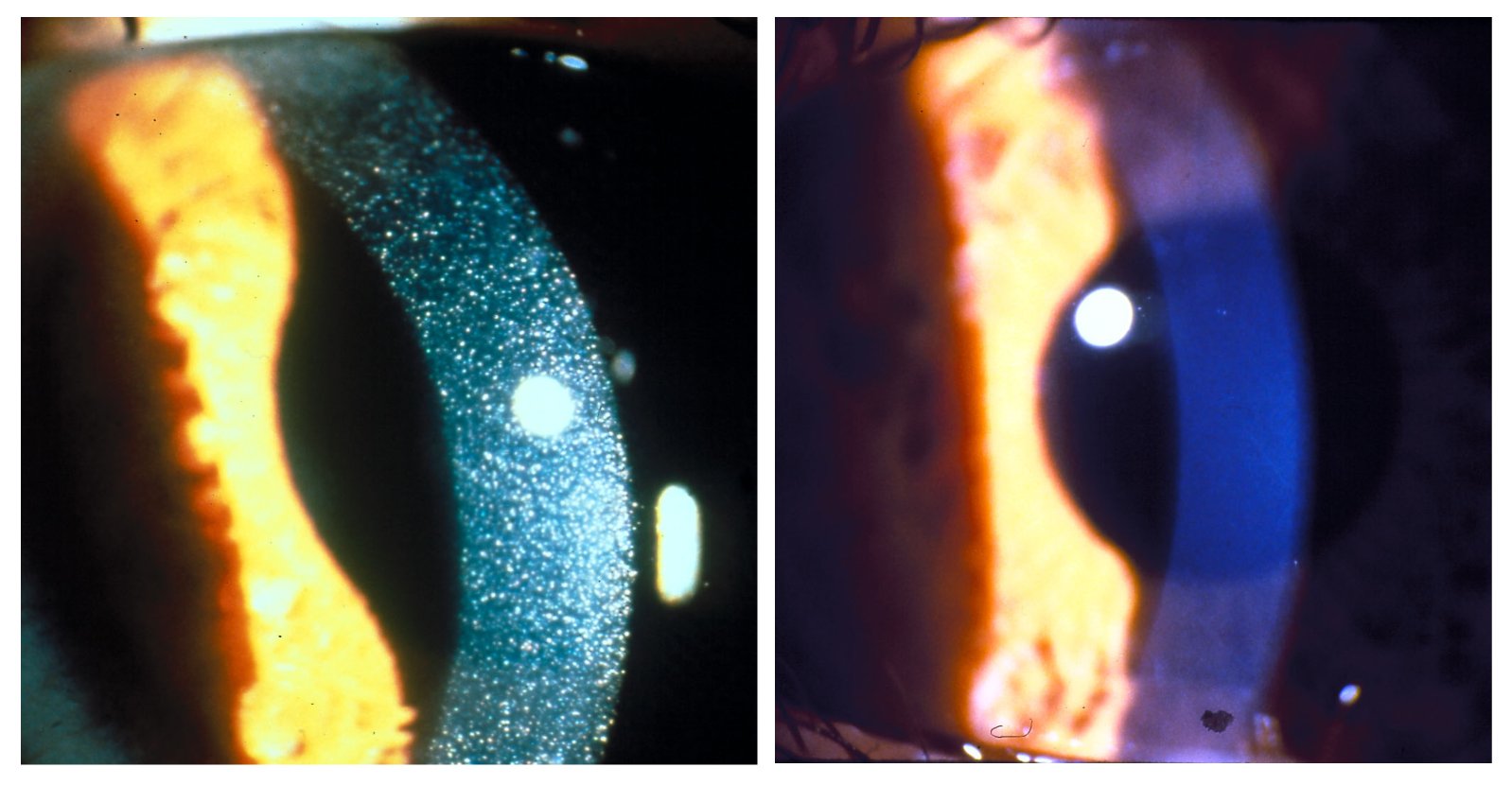
Cystinosis
Cystinosis is a lysosomal storage disease characterized by the abnormal accumulation of cystine, the oxidized dimer of the amino acid cysteine. It is a genetic disorder that follows an autosomal recessive inheritance pattern. It is a rare autosomal recessive disorder resulting from accumulation of free cystine in lysosomes, eventually leading to intracellular crystal formation throughout the body. Cystinosis is the most common cause of Fanconi syndrome in the pediatric age group. Fanconi syndrome occurs when the function of cells in renal tubules is impaired, leading to abnormal amounts of carbohydrates and amino acids in the urine, excessive urination, and low blood levels of potassium and phosphates. Cystinosis was the first documented genetic disease belonging to the group of lysosomal storage disease disorders.Nesterova G, Gahl WA. Cystinosis: the evolution of a treatable disease. Pediatr Nephrol 2012;28:51–9. Cystinosis is caused by mutations in the '' CTNS'' gene that code ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disulfide Bond
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In biology, disulfide bridges formed between thiol groups in two cysteine residues are an important component of the secondary and tertiary structure of proteins. '' Persulfide'' usually refers to compounds. In inorganic chemistry disulfide usually refers to the corresponding anion (−S−S−). Organic disulfides Symmetrical disulfides are compounds of the formula . Most disulfides encountered in organo sulfur chemistry are symmetrical disulfides. Unsymmetrical disulfides (also called heterodisulfides) are compounds of the formula . They are less common in organic chemistry, but most disulfides in nature are unsymmetrical. Properties The disulfide bonds are strong, with a typical bond dissociation energy of 60 kcal/mol (251&n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile. When present as a deprotonated catalytic residue, sometimes the symbol Cyz is used. The deprotonated form can generally be described by the symbol Cym as well. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. In this case, the symbol Cyx is sometimes used. When used as a food additive, it has the E number E920. Cysteine is encoded by the codons UGU and UGC. The sulfur-containing amino acids cysteine and methionine are more easily oxidized than the other amino acids. Structure Like other amino acids (not as a residue of a protein), cysteine exists as a zwitterion. Cysteine has chirality in the older / notation based on homology to - and -glyceraldehyde. In the newer ''R''/''S'' system of design ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disulfide
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In biology, disulfide bridges formed between thiol groups in two cysteine residues are an important component of the secondary and tertiary structure of proteins. '' Persulfide'' usually refers to compounds. In inorganic chemistry disulfide usually refers to the corresponding anion (−S−S−). Organic disulfides Symmetrical disulfides are compounds of the formula . Most disulfides encountered in organo sulfur chemistry are symmetrical disulfides. Unsymmetrical disulfides (also called heterodisulfides) are compounds of the formula . They are less common in organic chemistry, but most disulfides in nature are unsymmetrical. Properties The disulfide bonds are strong, with a typical bond dissociation energy of 60 kcal/mol (251&n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthionine
Lanthionine is a nonproteinogenic amino acid with the chemical formula (HOOC-CH(NH2)-CH2-S-CH2-CH(NH2)-COOH). It is typically formed by a cysteine residue and a dehydrated serine residue. Despite its name, lanthionine does not contain the element lanthanum. Background In 1941, lanthionine was first isolated by treating wool with sodium carbonate. It was found to be a sulfur-containing amino acid; accordingly it was given the name lanthionine ool (Latin: ''Lana''), sulfur (Greek: ''theîon'') Lanthionine was first synthesized by alkylation of cysteine with β- chloroalanine. Lanthionines are found widely in nature. They have been isolated from human hair, lactalbumin, and feathers. Lanthionines have also been found in bacterial cell walls and are the components of a group of gene-encoded peptide antibiotics called lantibiotics, which includes nisin (a food preservative), subtilin, epidermin (effective against ''Staphylococcus'' and ''Streptococcus''), and ancovenin (an enzy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylcysteine
Acetylcysteine, also known as ''N''-acetylcysteine (NAC), is a medication that is used to treat paracetamol overdose and to loosen thick mucus in individuals with chronic bronchopulmonary disorders like pneumonia and bronchitis. It has been used to treat lactobezoar in infants. It can be taken intravenously, by mouth, or inhaled as a mist. Some people use it as a dietary supplement. Common side effects include nausea and vomiting when taken by mouth. The skin may occasionally become red and itchy with any route of administration. A non-immune type of anaphylaxis may also occur. It appears to be safe in pregnancy. For paracetamol overdose, it works by increasing the level of glutathione, an antioxidant that can neutralise the toxic breakdown products of paracetamol. When inhaled, it acts as a mucolytic by decreasing the thickness of mucus. Acetylcysteine was initially patented in 1960 and came into medical use in 1968. It is on the World Health Organization's List of Ess ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Emil Erlenmeyer
Richard August Carl Emil Erlenmeyer (28 June 182522 January 1909), known simply as Emil Erlenmeyer, was a German chemist known for contributing to the early development of the theory of structure, formulating the Erlenmeyer rule, and designing the Erlenmeyer flask, a type of specialized flask, ubiquitous in chemistry laboratories, which is named after him. Biography Earlyl life and education Erlenmeyer was born in Wehen, Duchy of Nassau (today Taunusstein, Hesse, near Wiesbaden), in 1825, the son of a Protestant minister. He enrolled in the University of Giessen to study medicine, but after attending lectures of Justus von Liebig changed to chemistry. In the summer of 1846 he went to Heidelberg for one year, and studied physics, botany and mineralogy, returning to Giessen in 1847. After serving as assistant to H. Will and then to Carl Remigius Fresenius, Erlenmeyer decided to devote himself to pharmaceutical chemistry. For this purpose he studied in Nassau, where he passe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dithiothreitol
Dithiothreitol (DTT) is the common name for a small-molecule redox reagent also known as Cleland's reagent, after W. Wallace Cleland. DTT's formula is C4H10O2S2 and the chemical structure of one of its enantiomers in its reduced form is shown on the right; its oxidized form is a disulfide bonded 6-membered ring (shown below). The reagent is commonly used in its racemic form, as both enantiomers are reactive. Its name derives from the four-carbon sugar, threose. DTT has an epimeric ('sister') compound, dithioerythritol (DTE). Reducing agent DTT is a reducing agent; once oxidized, it forms a stable six-membered ring with an internal disulfide bond. It has a redox potential of −0.33 V at pH 7. The reduction of a typical disulfide bond proceeds by two sequential thiol-disulfide exchange reactions and is illustrated below. The reduction usually does not stop at the mixed-disulfide species because the second thiol of DTT has a high propensity to close the ring, forming oxidized ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercaptoethanol
2-Mercaptoethanol (also β-mercaptoethanol, BME, 2BME, 2-ME or β-met) is the chemical compound with the formula HOCH2CH2SH. ME or βME, as it is commonly abbreviated, is used to reduce disulfide bonds and can act as a biological antioxidant by scavenging hydroxyl radicals (amongst others). It is widely used because the hydroxyl group confers solubility in water and lowers the volatility. Due to its diminished vapor pressure, its odor, while unpleasant, is less objectionable than related thiols. Production 2-Mercaptoethanol is manufactured industrially by the reaction of ethylene oxide with hydrogen sulfide. Thiodiglycol and various zeolites catalyze the reaction. : Reactions 2-Mercaptoethanol reacts with aldehydes and ketones to give the corresponding oxathiolanes. This makes 2-mercaptoethanol useful as a protecting group, giving a derivative whose stability is between that of a dioxolane and a dithiolane. : Applications Reducing proteins Some proteins can be denat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |