|
Coordination Geometry
The term coordination geometry is used in a number of related fields of chemistry and solid state chemistry/physics. Molecules The coordination geometry of an atom is the geometrical pattern formed by atoms around the central atom. Inorganic coordination complexes In the field of inorganic coordination complexes it is the geometrical pattern formed by the atoms in the ligands that are bonded to the central atom in a molecule or a coordination complex. The geometrical arrangement will vary according to the number and type of ligands bonded to the metal centre, and to the coordination preference of the central atom, typically a metal in a coordination complex. The number of atoms bonded, (i.e. the number of σ-bonds between central atom and ligands) is termed the coordination number. The geometrical pattern can be described as a polyhedron where the vertices of the polyhedron are the centres of the coordinating atoms in the ligands. The coordination preference of a metal often va ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environmental chemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Sphere
In coordination chemistry, the first coordination sphere refers to the array of molecules and ions (the ligands) directly attached to the central metal atom. The second coordination sphere consists of molecules and ions that attached in various ways to the first coordination sphere. First coordination sphere The first coordination sphere refers to the molecules that are attached directly to the metal. The interactions between the first and second coordination spheres usually involve hydrogen-bonding. For charged complexes, ion pairing is important. In hexamminecobalt(III) chloride ( o(NH3)6l3), the cobalt cation plus the 6 ammonia ligands comprise the first coordination sphere. The coordination sphere of this ion thus consists of a central MN6 core "decorated" by 18 N−H bonds that radiate outwards. Second coordination sphere Metal ions can be described as consisting of series of two concentric coordination spheres, the first and second. More distant from the second coo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
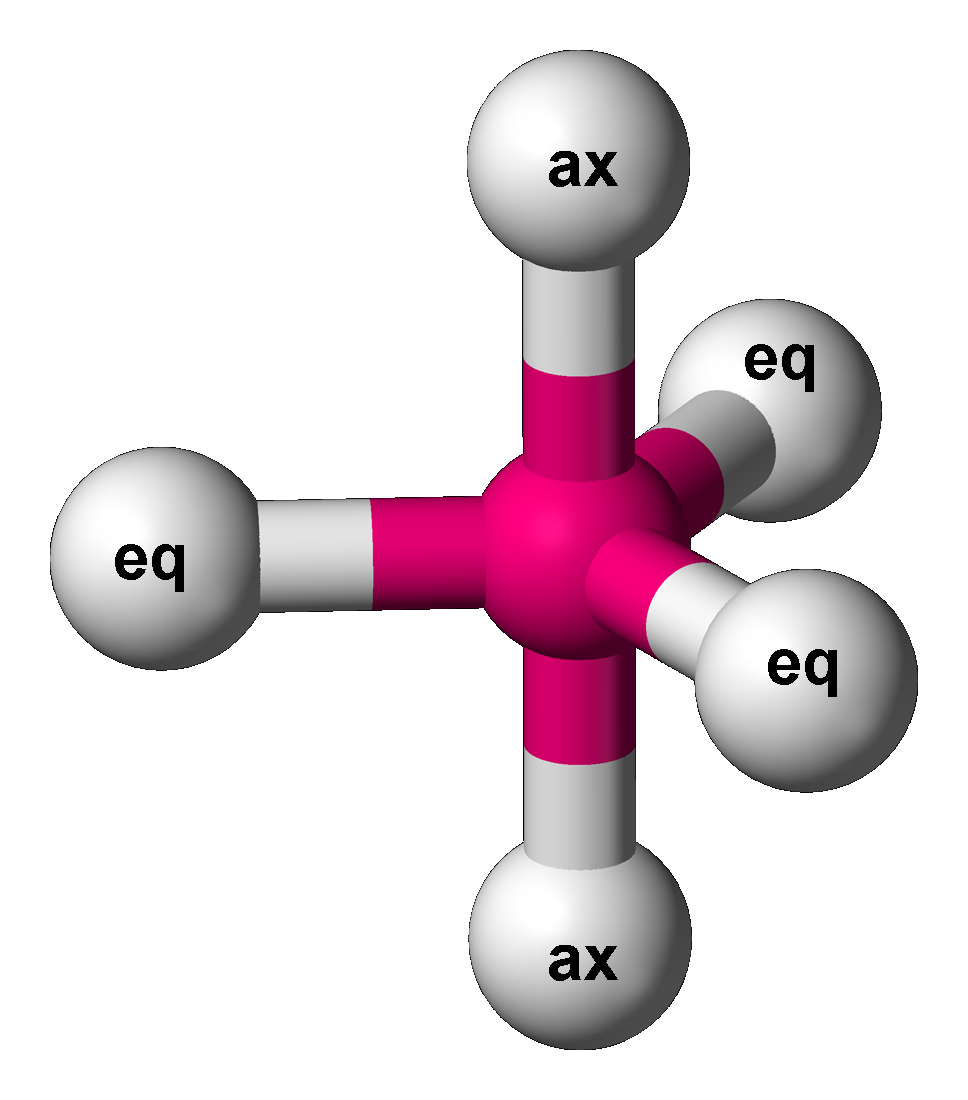
Trigonal Bipyramidal Molecular Geometry
In chemistry, a trigonal bipyramid formation is a molecular geometry with one atom at the center and 5 more atoms at the corners of a triangular bipyramid. This is one geometry for which the bond angles surrounding the central atom are not identical (see also pentagonal bipyramid), because there is no geometrical arrangement with five terminal atoms in equivalent positions. Examples of this molecular geometry are phosphorus pentafluoride (), and phosphorus pentachloride () in the gas phase. Axial (or apical) and equatorial positions The five atoms bonded to the central atom are not all equivalent, and two different types of position are defined. For phosphorus pentachloride as an example, the phosphorus atom shares a plane with three chlorine atoms at 120° angles to each other in ''equatorial'' positions, and two more chlorine atoms above and below the plane (''axial'' or ''apical'' positions). According to the VSEPR theory of molecular geometry, an axial position is more crowd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper(II) Oxide
Copper(II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu2O or copper(I) oxide (cuprous oxide). As a mineral, it is known as tenorite. It is a product of copper mining and the precursor to many other copper-containing products and chemical compounds. Production It is produced on a large scale by pyrometallurgy, as one stage in extracting copper from its ores. The ores are treated with an aqueous mixture of ammonium carbonate, ammonia, and oxygen to give copper(I) and copper(II) ammine complexes, which are extracted from the solids. These complexes are decomposed with steam to give CuO. It can be formed by heating copper in air at around 300–800°C: : 2 Cu + O2 → 2 CuO For laboratory uses, pure copper(II) oxide is better prepared by heating copper(II) nitrate, copper(II) hydroxide, or basic copper(II) carbonate: : 2 Cu(NO3)2(s) → 2 CuO(s) + 4 NO2(g) + O2(g) (180° ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon Dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and most abundant families of materials, existing as a compound of several minerals and as a synthetic product. Notable examples include fused quartz, fumed silica, silica gel, opal and aerogels. It is used in structural materials, microelectronics (as an electrical insulator), and as components in the food and pharmaceutical industries. Structure In the majority of silicates, the silicon atom shows tetrahedral coordination, with four oxygen atoms surrounding a central Si atomsee 3-D Unit Cell. Thus, SiO2 forms 3-dimensional network solids in which each silicon atom is covalently bonded in a tetrahedral manner to 4 oxygen atoms. In contrast, CO2 is a linear molecule. The starkly different structures of the dioxid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
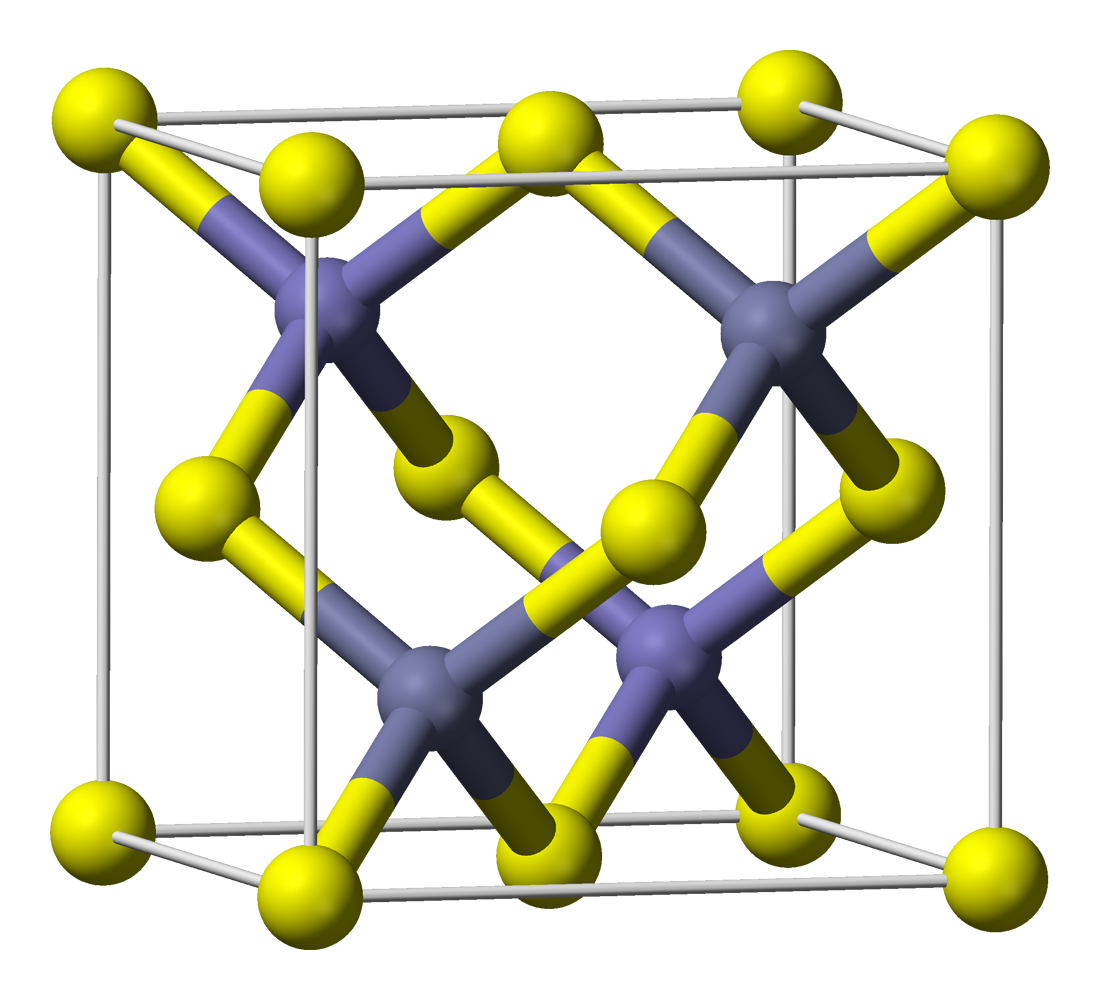
Zinc Sulfide
Zinc sulfide (or zinc sulphide) is an inorganic compound with the chemical formula of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite. Although this mineral is usually black because of various impurities, the pure material is white, and it is widely used as a pigment. In its dense synthetic form, zinc sulfide can be transparent, and it is used as a window for visible optics and infrared optics. Structure ZnS exists in two main crystalline forms. This dualism is an example of polymorphism. In each form, the coordination geometry at Zn and S is tetrahedral. The more stable cubic form is known also as zinc blende or sphalerite. The hexagonal form is known as the mineral wurtzite, although it also can be produced synthetically.. The transition from the sphalerite form to the wurtzite form occurs at around 1020 °C. A tetragonal form is also known as the very rare mineral called polhemusite, with the formula . Applicatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium Dioxide
Titanium dioxide, also known as titanium(IV) oxide or titania , is the inorganic compound with the chemical formula . When used as a pigment, it is called titanium white, Pigment White 6 (PW6), or CI 77891. It is a white solid that is insoluble to water, although mineral forms can appear black. As a pigment, it has a wide range of applications, including paint, sunscreen, and food coloring. When used as a food coloring, it has E number E171. World production in 2014 exceeded 9 million tonnes. It has been estimated that titanium dioxide is used in two-thirds of all pigments, and pigments based on the oxide have been valued at a price of $13.2 billion. Structure In all three of its main dioxides, titanium exhibits octahedral geometry, being bonded to six oxide anions. The oxides in turn are bonded to three Ti centers. The overall crystal structure of rutile is tetragonal in symmetry whereas anatase and brookite are orthorhombic. The oxygen substructures are all slight distort ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trigonal Planar Molecular Geometry
In chemistry, trigonal planar is a molecular geometry model with one atom at the center and three atoms at the corners of an equilateral triangle, called peripheral atoms, all in one plane. In an ideal trigonal planar species, all three ligands are identical and all bond angles are 120°. Such species belong to the point group D3h. Molecules where the three ligands are not identical, such as H2CO, deviate from this idealized geometry. Examples of molecules with trigonal planar geometry include boron trifluoride (BF3), formaldehyde (H2CO), phosgene (COCl2), and sulfur trioxide (SO3). Some ions with trigonal planar geometry include nitrate (), carbonate (), and guanidinium (). In organic chemistry, planar, three-connected carbon centers that are trigonal planar are often described as having sp2 hybridization. Nitrogen inversion is the distortion of pyramidal amines through a transition state that is trigonal planar. Pyramidalization is a distortion of this molecular shape t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

4-3D-balls.png)