|
Concentration Polarization
Concentration polarization is a term used in the scientific fields of electrochemistry and membrane science. In electrochemistry In electrochemistry, concentration polarization denotes the part of the polarization of an electrolytic cell resulting from changes in the electrolyte concentration due to the passage of current through the electrode/solution interface. Here ''polarization'' is understood as the shift of the electrochemical potential difference across the cell from its equilibrium value. When the term is used in this sense, it is equivalent to “ concentration overpotential”. the changes in concentration (emergence of concentration gradients in the solution adjacent to the electrode surface) is the difference in the rate of electrochemical reaction at the electrode and the rate of ion migration in the solution from/to the surface. When a chemical species participating in an electrochemical electrode reaction is in short supply, the concentration of this species at the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outcome of a particular chemical change, or vice versa. These reactions involve electrons moving via an electronically-conducting phase (typically an external electrical circuit, but not necessarily, as in electroless plating) between electrodes separated by an ionically conducting and electronically insulating electrolyte (or ionic species in a solution). When a chemical reaction is driven by an electrical potential difference, as in electrolysis, or if a potential difference results from a chemical reaction as in an electric battery or fuel cell, it is called an ''electrochemical'' reaction. Unlike in other chemical reactions, in electrochemical reactions electrons are not transferred directly between atoms, ions, or molecules, but via ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ultrafiltration
Ultrafiltration (UF) is a variety of membrane filtration in which forces such as pressure or concentration gradients lead to a separation through a semipermeable membrane. Suspended solids and solutes of high molecular weight are retained in the so-called retentate, while water and low molecular weight solutes pass through the membrane in the permeate (filtrate). This separation process is used in industry and research for purifying and concentrating macromolecular (103–106 Da) solutions, especially protein solutions. Ultrafiltration is not fundamentally different from microfiltration. Both of these separate based on size exclusion or particle capture. It is fundamentally different from membrane gas separation, which separate based on different amounts of absorption and different rates of diffusion. Ultrafiltration membranes are defined by the molecular weight cut-off (MWCO) of the membrane used. Ultrafiltration is applied in cross-flow or dead-end mode. Applicat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Desalination
Desalination is a process that takes away mineral components from saline water. More generally, desalination refers to the removal of salts and minerals from a target substance, as in soil desalination, which is an issue for agriculture. Saltwater (especially sea water) is desalinated to produce water suitable for human consumption or irrigation. The by-product of the desalination process is brine. Desalination is used on many seagoing ships and submarines. Most of the modern interest in desalination is focused on cost-effective provision of fresh water for human use. Along with recycled wastewater, it is one of the few rainfall-independent water resources. Due to its energy consumption, desalinating sea water is generally more costly than fresh water from surface water or groundwater, water recycling and water conservation. However, these alternatives are not always available and depletion of reserves is a critical problem worldwide. Desalination processes are usin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
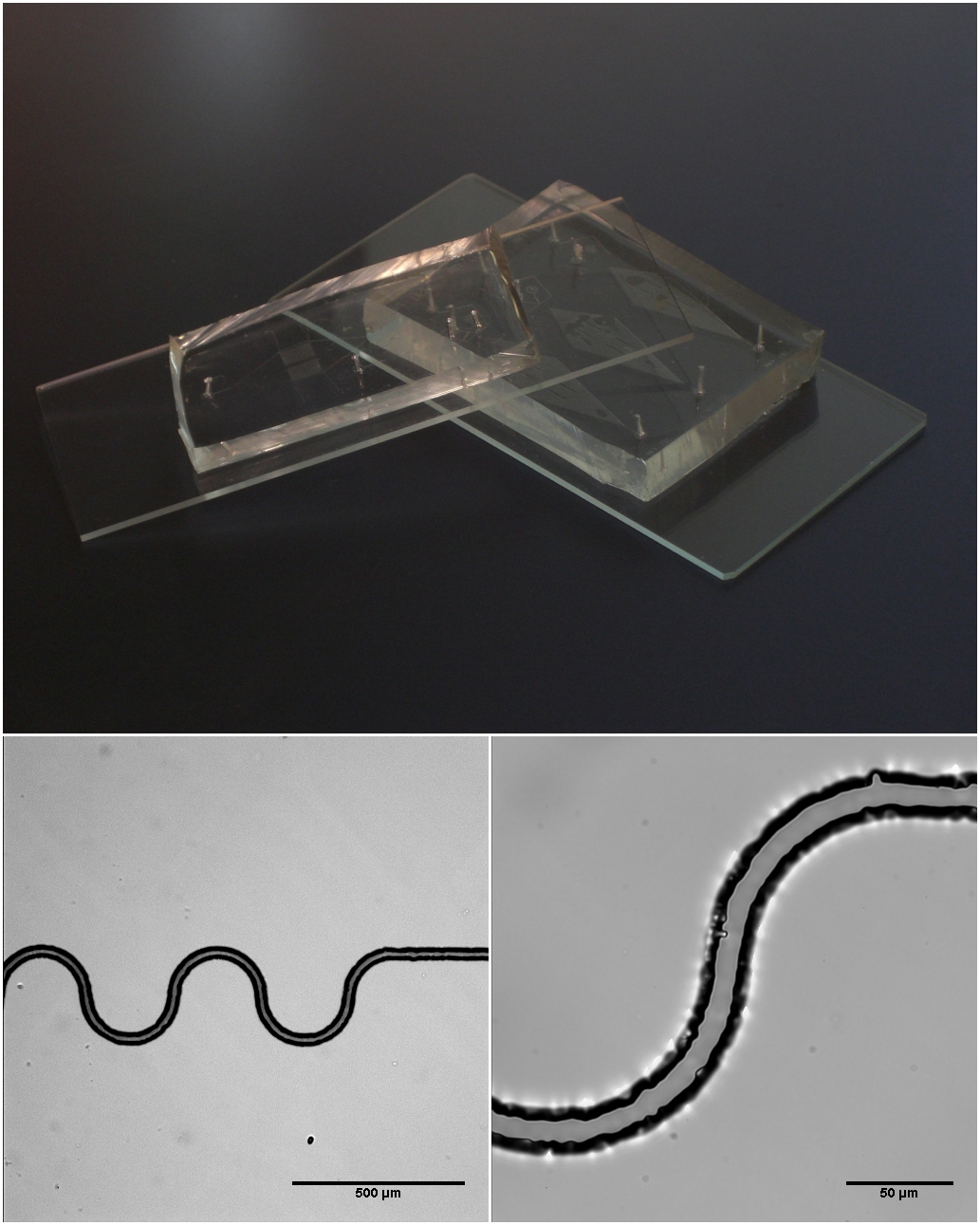
Microfluidics
Microfluidics refers to the behavior, precise control, and manipulation of fluids that are geometrically constrained to a small scale (typically sub-millimeter) at which surface forces dominate volumetric forces. It is a multidisciplinary field that involves engineering, physics, chemistry, biochemistry, nanotechnology, and biotechnology. It has practical applications in the design of systems that process low volumes of fluids to achieve multiplexing, automation, and high-throughput screening. Microfluidics emerged in the beginning of the 1980s and is used in the development of inkjet printheads, DNA chips, lab-on-a-chip technology, micro-propulsion, and micro-thermal technologies. Typically, micro means one of the following features: * Small volumes (μL, nL, pL, fL) * Small size * Low energy consumption * Microdomain effects Typically microfluidic systems transport, mix, separate, or otherwise process fluids. Various applications rely on passive fluid control using capillary ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microfluidic
Microfluidics refers to the behavior, precise control, and manipulation of fluids that are geometrically constrained to a small scale (typically sub-millimeter) at which surface forces dominate volumetric forces. It is a multidisciplinary field that involves engineering, physics, chemistry, biochemistry, nanotechnology, and biotechnology. It has practical applications in the design of systems that process low volumes of fluids to achieve multiplexing, automation, and high-throughput screening. Microfluidics emerged in the beginning of the 1980s and is used in the development of inkjet printheads, DNA chips, lab-on-a-chip technology, micro-propulsion, and micro-thermal technologies. Typically, micro means one of the following features: * Small volumes (μL, nL, pL, fL) * Small size * Low energy consumption * Microdomain effects Typically microfluidic systems transport, mix, separate, or otherwise process fluids. Various applications rely on passive fluid control using capillary f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrokinetic Phenomena
Electrokinetic phenomena are a family of several different effects that occur in heterogeneous fluids, or in porous bodies filled with fluid, or in a fast flow over a flat surface. The term heterogeneous here means a fluid containing particles. Particles can be solid, liquid or gas bubbles with sizes on the scale of a micrometer or nanometer.Dukhin, A. S. and Goetz, P. J. ''Characterization of liquids, nano- and micro- particulates and porous bodies using Ultrasound'', Elsevier, 2017 There is a common source of all these effects—the so-called interfacial 'double layer' of charges. Influence of an external force on the diffuse layer generates tangential motion of a fluid with respect to an adjacent charged surface. This force might be electric, pressure gradient, concentration gradient, or gravity. In addition, the moving phase might be either continuous fluid or dispersed phase. Family Various combinations of the driving force and moving phase determine various electrokinetic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fouling
Fouling is the accumulation of unwanted material on solid surfaces. The fouling materials can consist of either living organisms ( biofouling) or a non-living substance (inorganic or organic). Fouling is usually distinguished from other surface-growth phenomena in that it occurs on a surface of a component, system, or plant performing a defined and useful function and that the fouling process impedes or interferes with this function. Other terms used in the literature to describe fouling include deposit formation, encrustation, crudding, deposition, scaling, scale formation, slagging, and sludge formation. The last six terms have a more narrow meaning than fouling within the scope of the fouling science and technology, and they also have meanings outside of this scope; therefore, they should be used with caution. Fouling phenomena are common and diverse, ranging from fouling of ship hulls, natural surfaces in the marine environment ( marine fouling), fouling of heat-transf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osmotic Pressure
Osmotic pressure is the minimum pressure which needs to be applied to a solution to prevent the inward flow of its pure solvent across a semipermeable membrane. It is also defined as the measure of the tendency of a solution to take in a pure solvent by osmosis. Potential osmotic pressure is the maximum osmotic pressure that could develop in a solution if it were separated from its pure solvent by a semipermeable membrane. Osmosis occurs when two solutions containing different concentrations of solute are separated by a selectively permeable membrane. Solvent molecules pass preferentially through the membrane from the low-concentration solution to the solution with higher solute concentration. The transfer of solvent molecules will continue until equilibrium is attained. Theory and measurement Jacobus van 't Hoff found a quantitative relationship between osmotic pressure and solute concentration, expressed in the following equation: :\Pi = icRT where \Pi is osmotic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Limiting Current Density
In electronics, a limiter is a circuit that allows signals below a specified input power or level to pass unaffected while attenuating (lowering) the peaks of stronger signals that exceed this threshold. Limiting is a type of dynamic range compression. Clipping is an extreme version of limiting. Limiting is any process by which the amplitude of a signal is prevented from exceeding a predetermined value. Limiters are common as a safety device in live sound and broadcast applications to prevent sudden volume peaks from occurring. Limiters are also used as protective features in some components of sound reinforcement systems (e.g., powered mixing boards and power amplifiers) and in some bass amplifiers, to prevent unwanted distortion or loudspeaker damage. Types Limiting can refer to a range of treatments designed to limit the maximum level of a signal. Treatments in order of decreasing severity range from clipping, in which a signal is passed through normally but sheared off wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrodialysis
Electrodialysis (ED) is used to transport salt ions from one solution through ion-exchange membranes to another solution under the influence of an applied electric potential difference. This is done in a configuration called an electrodialysis cell. The cell consists of a feed (dilute) compartment and a concentrate (brine) compartment formed by an anion exchange membrane and a cation exchange membrane placed between two electrodes. In almost all practical electrodialysis processes, multiple electrodialysis cells are arranged into a configuration called an electrodialysis stack, with alternating anion and cation-exchange membranes forming the multiple electrodialysis cells. Electrodialysis processes are different from distillation techniques and other membrane based processes (such as reverse osmosis (RO)) in that dissolved species are moved away from the feed stream rather than the reverse. Because the quantity of dissolved species in the feed stream is far less than that o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kidney Dialysis
Kidney dialysis (from Greek , , 'dissolution'; from , , 'through', and , , 'loosening or splitting') is the process of removing excess water, solutes, and toxins from the blood in people whose kidneys can no longer perform these functions naturally. This is referred to as renal replacement therapy. The first successful dialysis was performed in 1943. Dialysis may need to be initiated when there is a sudden rapid loss of kidney function, known as acute kidney injury (previously called acute renal failure), or when a gradual decline in kidney function, chronic kidney disease, reaches stage 5. Stage 5 chronic renal failure is reached when the glomerular filtration rate is 10–15% of normal, creatinine clearance is less than 10 mL per minute and uremia is present. Dialysis is used as a temporary measure in either acute kidney injury or in those awaiting kidney transplant and as a permanent measure in those for whom a transplant is not indicated or not possible.Pendse S, Sin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microfiltration
Microfiltration is a type of physical filtration process where a contaminated fluid is passed through a special pore-sized membrane filter to separate microorganisms and suspended particles from process liquid. It is commonly used in conjunction with various other separation processes such as ultrafiltration and reverse osmosis to provide a product stream which is free of undesired contaminants. General principles Microfiltration usually serves as a pre-treatment for other separation processes such as ultrafiltration, and a post-treatment for granular media filtration. The typical particle size used for microfiltration ranges from about 0.1 to 10 μm.Baker, R 2012, ''Microfiltration, in Membrane Technology and Applications'', 3rd edn, John Wiley & Sons Ltd, California. p. 303 In terms of approximate molecular weight these membranes can separate macromolecules of molecular weights generally less than 100,000 g/mol.Microfiltration/Ultrafiltration, 2008, Hyflux Membranes, access ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |