|
Colitose
Colitose is a mannose-derived 3,6-dideoxysugar produced by certain bacteria. It is a constituent of the lipopolysaccharide. It is the enantiomer of abequose. Biological role Colitose is found in the O-antigen of certain Gram-negative bacteria such as ''Escherichia coli'', ''Yersinia pseudotuberculosis'', ''Salmonella enterica'', ''Vibrio cholerae'', and in marine bacteria such as ''Pseudoalteromonas sp''. The sugar was first isolated in 1958, and subsequently was enzymatically synthesized in 1962. Biosynthesis The biosynthesis of colitose begins with ColE, a mannose-1-phosphate guanylyltransferase that catalyzes the addition of a GMP moiety to mannose, yielding GDP-mannose. In the next step, ColB, an NADP-dependent short-chain dehydrogenase-reductase enzyme, catalyzes the oxidation at C-4 and the removal of the hydroxyl group at C-6. The resulting product, GDP-4-keto-6-deoxymannose, then reacts with the PLP-dependent enzyme GDP-4-keto-6-deoxymannose-3-dehydratase (ColD), wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deoxy Sugars
Deoxy sugars are sugars that have had a hydroxyl group replaced with a hydrogen atom. Examples include: * Deoxyribose, or 2-deoxy-D-ribose, a constituent of DNA * Fucose, or 6-deoxy-L-galactose, main component of fucoidan of brown algae, and present in N-linked glycans * Fuculose, or 6-deoxy-L-tagatose, one of the important components of avian influenza virus particles * Rhamnose, or 6-deoxy-L-mannose, present in plant glycosides In ''Escherichia coli'' bacteria, deoxyribose sugars are synthesized via two different pathways - one pathway involves aldol condensation, whereas the other pathway is conversion of a ribose sugar into a deoxyribose sugar by means of changes on the nucleotide or nucleoside level. Deoxyribose is synthesized through the reduction of ribose. Deoxyribose is derived from the same precursor as ribose being that the reduction of the sugar with the extra hydroxyl group results in the deoxy-sugar, which has its hydroxyl group replaced with a hydrogen atom. Di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dideoxy Sugar
Deoxy sugars are sugars that have had a hydroxyl group replaced with a hydrogen atom. Examples include: * Deoxyribose, or 2-deoxy-D-ribose, a constituent of DNA * Fucose, or 6-deoxy-L-galactose, main component of fucoidan of brown algae, and present in N-linked glycans * Fuculose, or 6-deoxy-L-tagatose, one of the important components of avian influenza virus particles * Rhamnose, or 6-deoxy-L-mannose, present in plant glycosides In ''Escherichia coli'' bacteria, deoxyribose sugars are synthesized via two different pathways - one pathway involves aldol condensation, whereas the other pathway is conversion of a ribose sugar into a deoxyribose sugar by means of changes on the nucleotide or nucleoside level. Deoxyribose is synthesized through the reduction of ribose. Deoxyribose is derived from the same precursor as ribose being that the reduction of the sugar with the extra hydroxyl group results in the deoxy-sugar, which has its hydroxyl group replaced with a hydrogen atom. Did ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
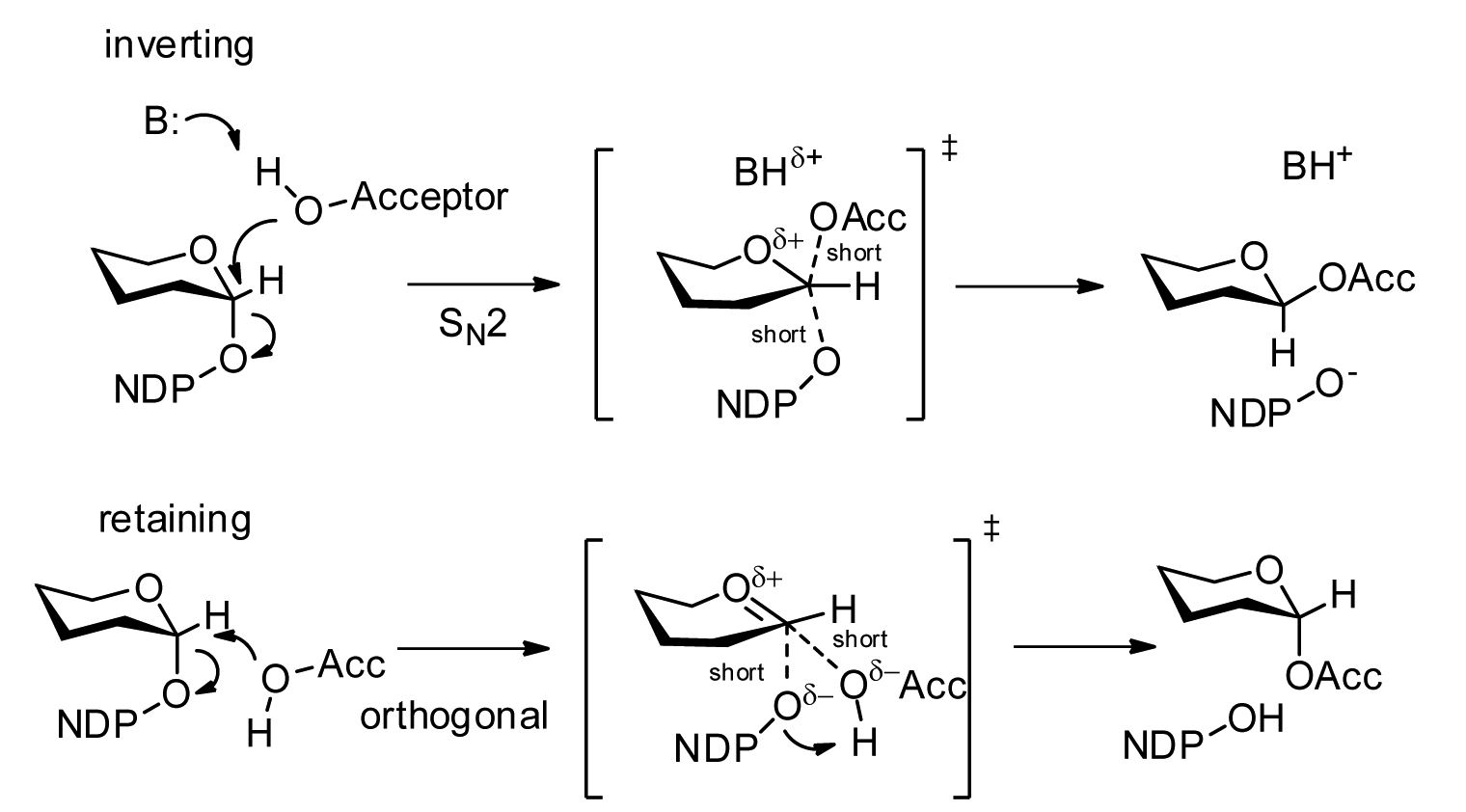
Glycosyltransferase
Glycosyltransferases (GTFs, Gtfs) are enzymes ( EC 2.4) that establish natural glycosidic linkages. They catalyze the transfer of saccharide moieties from an activated nucleotide sugar (also known as the "glycosyl donor") to a nucleophilic glycosyl acceptor molecule, the nucleophile of which can be oxygen- carbon-, nitrogen-, or sulfur-based. The result of glycosyl transfer can be a carbohydrate, glycoside, oligosaccharide, or a polysaccharide. Some glycosyltransferases catalyse transfer to inorganic phosphate or water. Glycosyl transfer can also occur to protein residues, usually to tyrosine, serine, or threonine to give O-linked glycoproteins, or to asparagine to give N-linked glycoproteins. Mannosyl groups may be transferred to tryptophan to generate C-mannosyl tryptophan, which is relatively abundant in eukaryotes. Transferases may also use lipids as an acceptor, forming glycolipids, and even use lipid-linked sugar phosphate donors, such as dolichol phosphates in eukaryotic o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosyltransferases
Glycosyltransferases (GTFs, Gtfs) are enzymes ( EC 2.4) that establish natural glycosidic linkages. They catalyze the transfer of saccharide moieties from an activated nucleotide sugar (also known as the "glycosyl donor") to a nucleophilic glycosyl acceptor molecule, the nucleophile of which can be oxygen- carbon-, nitrogen-, or sulfur-based. The result of glycosyl transfer can be a carbohydrate, glycoside, oligosaccharide, or a polysaccharide. Some glycosyltransferases catalyse transfer to inorganic phosphate or water. Glycosyl transfer can also occur to protein residues, usually to tyrosine, serine, or threonine to give O-linked glycoproteins, or to asparagine to give N-linked glycoproteins. Mannosyl groups may be transferred to tryptophan to generate C-mannosyl tryptophan, which is relatively abundant in eukaryotes. Transferases may also use lipids as an acceptor, forming glycolipids, and even use lipid-linked sugar phosphate donors, such as dolichol phosphates in eukaryotic o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine Dehydratase
Serine dehydratase or L-serine ammonia lyase (SDH) is in the β-family of pyridoxal phosphate-dependent (PLP) enzymes. SDH is found widely in nature, but its structural and properties vary among species. SDH is found in yeast, bacteria, and the cytoplasm of mammalian hepatocytes. SDH catalyzes is the deamination of L-serine to yield pyruvate, with the release of ammonia. This enzyme has one substrate, L-serine, and two products, pyruvate and NH3, and uses one cofactor, pyridoxal phosphate (PLP). The enzyme's main role is in gluconeogenesis in the liver's cytoplasm. Nomenclature Serine Dehydratase is also known as: * L-serine ammonia-lyase * Serine deaminase * L-hydroxyaminoacid dehydratase * L-serine deaminase * L-serine dehydratase * L-serine hydro-lyase Structure The holoenzyme SDH contains 319 residues, one PLP cofactor molecule. The overall fold of the monomer is very similar to that of other PLP-dependent enzymes of the Beta-family. The enzyme contains a large c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridoxal-phosphate
Pyridoxal phosphate (PLP, pyridoxal 5'-phosphate, P5P), the active form of vitamin B6, is a coenzyme in a variety of enzymatic reactions. The International Union of Biochemistry and Molecular Biology has catalogued more than 140 PLP-dependent activities, corresponding to ~4% of all classified activities. The versatility of PLP arises from its ability to covalently bind the substrate, and then to act as an electrophilic catalyst, thereby stabilizing different types of carbanionic reaction intermediates. Role as a coenzyme PLP acts as a coenzyme in all transamination reactions, and in certain decarboxylation, deamination, and racemization reactions of amino acids. The aldehyde group of PLP forms a Schiff-base linkage (internal aldimine) with the ε-amino group of a specific lysine group of the aminotransferase enzyme. The α-amino group of the amino acid substrate displaces the ε-amino group of the active-site lysine residue in a process known as transaldimination. The resulting ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nicotinamide Adenine Dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme central to metabolism. Found in all living cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine nucleobase and the other nicotinamide. NAD exists in two forms: an oxidized and reduced form, abbreviated as NAD and NADH (H for hydrogen), respectively. In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The cofactor is, therefore, found in two forms in cells: NAD is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction, also with H+, forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, most notably as a substrate of enzymes in adding or removing chemical groups to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GDP-mannose
Guanosine diphosphate mannose or GDP-mannose is a nucleotide sugar that is a substrate for glycosyltransferase reactions in metabolism. This compound is a substrate for enzymes called mannosyltransferases. Known as donor of activated mannose in all glycolytic reactions, GDP-mannose is essential in eukaryotes. Biosynthesis GDP-mannose is produced from GTP and mannose-6-phosphate by the enzyme mannose-1-phosphate guanylyltransferase. One of the enzymes from the family of nucleootidyl-transferases, GDP-Mannose Pyrophosphorylase (GDP-MP) is an pervasive enzyme found in bacteria, fungi, plants, and animals. References See also * Nucleoside * Nucleotide * Guanosine * Guanosine diphosphate Guanosine diphosphate, abbreviated GDP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside guanosine. GDP consists of a pyrophosphate group, a pentose sugar ribose, and the nucleobase guanine. GDP is the product ... Nucleotides Coenzymes {{bioche ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Guanosine Monophosphate
Guanosine monophosphate (GMP), also known as 5′-guanidylic acid or guanylic acid (conjugate base guanylate), is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid with the nucleoside guanosine. GMP consists of the phosphate group, the pentose sugar ribose, and the nucleobase guanine; hence it is a ribonucleoside monophosphate. Guanosine monophosphate is commercially produced by microbial fermentation. As an acyl substituent, it takes the form of the prefix guanylyl-. ''De novo'' synthesis GMP synthesis starts with D-ribose 5′-phosphate, a product of the pentose phosphate pathway. The synthesis proceeds by the gradual formation of the purine ring on carbon-1 of ribose, with CO2, glutamine, glycine, aspartate and one-carbon derivatives of tetrahydrofolate donating various elements towards the building of the ring As inhibitor of guanosine monophosphate synthesis in experimental models, the glutamine analogue DON can be used.''Ahluwalia GS et alMet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mannose
Mannose is a sugar monomer of the aldohexose series of carbohydrates. It is a C-2 epimer of glucose. Mannose is important in human metabolism, especially in the glycosylation of certain proteins. Several congenital disorders of glycosylation are associated with mutations in enzymes involved in mannose metabolism. Mannose is not an essential nutrient; it can be produced in the human body from glucose, or converted into glucose. Mannose provides 2–5 kcal/g. It is partially excreted in the urine. Etymology The root of both "mannose" and "mannitol" is manna, which the Bible describes as the food supplied to the Israelites during their journey in the region of Sinai. Several trees and shrubs can produce a substance called manna, such as the "manna tree" ('' Fraxinus ornus'') from whose secretions mannitol was originally isolated. Structure Mannose commonly exists as two different-sized rings, the pyranose (six-membered) form and the furanose (five-membered) form. Eac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |