|
Cmin (pharmacology)
Cmin is a term used in pharmacokinetics for the minimum blood plasma concentration reached by a drug during the time interval between administration of two doses. This definition is slightly different from Ctrough, the concentration immediately prior to administration of the next dose. Cmin is the opposite of Cmax, the maximum concentration that the drug reaches. Cmin must be above certain thresholds, such as the minimum inhibitory concentration (MIC), to achieve a therapeutic effect. In most cases Cmin is directly measurable. At steady state the minimum plasma concentration can also be calculated using the following equation: :C_= \frac\times\ :''S'': salt factor :''F'': bioavailability :''D'': dose :''k'': elimination constant :''ka'': absorption constant :''Vd'': volume of distribution :''τ'': dosing interval Cmin is also an important parameter in bioavailability and bioequivalence Bioequivalence is a term in pharmacokinetics used to assess the expected in vivo biolog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmacokinetic
Pharmacokinetics (from Ancient Greek ''pharmakon'' "drug" and ''kinetikos'' "moving, putting in motion"; see chemical kinetics), sometimes abbreviated as PK, is a branch of pharmacology dedicated to determining the fate of substances administered to a living organism. The substances of interest include any chemical xenobiotic such as: pharmaceutical drugs, pesticides, food additives, cosmetics, etc. It attempts to analyze chemical metabolism and to discover the fate of a chemical from the moment that it is administered up to the point at which it is completely eliminated from the body. Pharmacokinetics is the study of how an organism affects a drug, whereas pharmacodynamics (PD) is the study of how the drug affects the organism. Both together influence dosing, benefit, and adverse effects, as seen in PK/PD models. Overview Pharmacokinetics describes how the body affects a specific xenobiotic/chemical after administration through the mechanisms of absorption and distribution, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blood Plasma
Blood plasma is a light amber-colored liquid component of blood in which blood cells are absent, but contains proteins and other constituents of whole blood in suspension. It makes up about 55% of the body's total blood volume. It is the intravascular part of extracellular fluid (all body fluid outside cells). It is mostly water (up to 95% by volume), and contains important dissolved proteins (6–8%; e.g., serum albumins, globulins, and fibrinogen), glucose, clotting factors, electrolytes (, , , , , etc.), hormones, carbon dioxide (plasma being the main medium for excretory product transportation), and oxygen. It plays a vital role in an intravascular osmotic effect that keeps electrolyte concentration balanced and protects the body from infection and other blood-related disorders. Blood plasma is separated from the blood by spinning a vessel of fresh blood containing an anticoagulant in a centrifuge until the blood cells fall to the bottom of the tube. The blood plasma is t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ctrough
In medicine and pharmacology, a trough level or trough concentration (Ctrough) is the concentration reached by a drug immediately before the next dose is administered,AGAH working group Pharmacokinetics, 2004. often used in . The name comes from the idea that on a graph of concentration versus time, the line forms a U-shaped trough at the lowest region, before a new dose sends it higher again. The usual criterion is concentration in the blood serum, although in some instances local concentration within [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CMax
CMAX is a bus rapid transit (BRT) service in Central Ohio, operated by the Central Ohio Transit Authority (COTA). The line begins in Downtown Columbus, traveling northeast to Westerville. CMAX is Central Ohio's first bus rapid transit line; it began operation in 2018. Route and fleet CMAX runs from Downtown Columbus to Westerville, primarily along Cleveland Avenue and High Street. The route is long. The trip is estimated to take about 56 minutes, depending on the time of day. Landmarks and parks along the line include Franklin University, the Franklin County Government Center, the South High Commercial Historic District, Columbus Commons, the Ohio Statehouse on Capitol Square, the High and Gay Streets Historic District, Sensenbrenner Park, Battelle Hall and the Greater Columbus Convention Center, the Central Ohio Fire Museum, Columbus State Community College, Fort Hayes, Mount Carmel St Ann's Hospital, Sharon Woods Metro Park, and the OhioHealth Westerville Medical Campus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
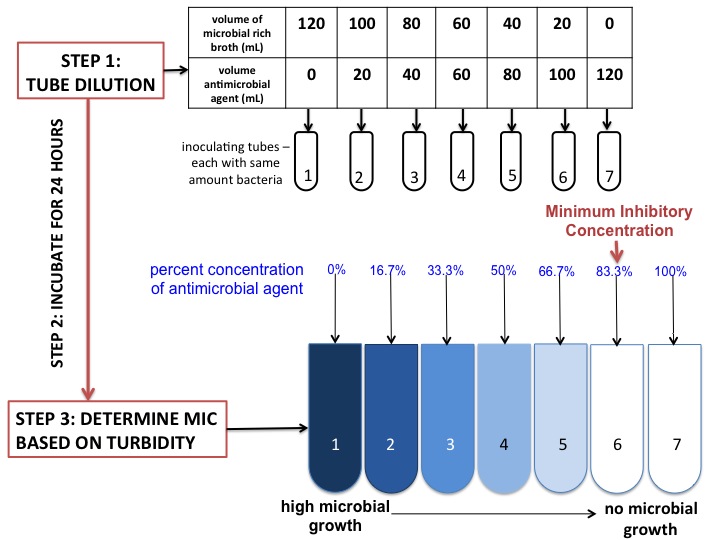
Minimum Inhibitory Concentration
In microbiology, the minimum inhibitory concentration (MIC) is the lowest concentration of a chemical, usually a drug, which prevents visible growth of a bacterium or bacteria. MIC depends on the microorganism, the affected human being (in vivo only), and the antibiotic itself. It is often expressed in micrograms per milliliter (μg/mL) or milligrams per liter (mg/L). The MIC is determined by preparing solutions of the chemical in vitro at increasing concentrations, incubating the solutions with separate batches of cultured bacteria, and measuring the results using agar dilution or broth microdilution. Results have been graded into susceptible (often called sensitive), increased exposure, or resistant to a particular antimicrobial by using a breakpoint. Breakpoints are agreed upon values, published in guidelines of a reference body, such as the U.S. Clinical and Laboratory Standards Institute (CLSI), the British Society for Antimicrobial Chemotherapy (BSAC) or the European Committee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Therapeutic Effect
Therapeutic effect refers to the response(s) after a treatment of any kind, the results of which are judged to be useful or favorable. This is true whether the result was expected, unexpected, or even an unintended consequence. An adverse effect (including nocebo) is the converse and refers to harmful or undesired response(s). What constitutes a therapeutic effect versus a side effect is a matter of both the nature of the situation and the goals of treatment. No inherent difference separates therapeutic and undesired side effects; both responses are behavioral/physiologic changes that occur as a response to the treatment strategy or agent. Treatment scope To maximize therapeutic effects (desired) and minimize side effects (undesired) requires recognition and quantification of the treatment in multiple dimensions. In the specific case of targeted pharmaceutical interventions, a combination of therapies is often needed to achieve the desired results. Pharmacology examples *A 201 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bioavailability
In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation. By definition, when a medication is administered intravenously, its bioavailability is 100%. However, when a medication is administered via routes other than intravenous, its bioavailability is generally lower than that of intravenous due to intestinal endothelium absorption and first-pass metabolism. Thereby, mathematically, bioavailability equals the ratio of comparing the area under the plasma drug concentration curve versus time (AUC) for the extravascular formulation to the AUC for the intravascular formulation. AUC is used because AUC is proportional to the dose that has entered the systemic circulation. Bioavailability of a drug is an average value; to take population variability into account, deviation range is shown as ±. To ensure that the drug taker who has poor absorption is dosed appropriately, the bottom value o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Volume Of Distribution
In pharmacology, the volume of distribution (VD, also known as apparent volume of distribution, literally, ''volume of dilution'') is the theoretical volume that would be necessary to contain the total amount of an administered drug at the same concentration that it is observed in the blood plasma. In other words, it is the ratio of ''amount of drug in a body (dose)'' to ''concentration of the drug that is measured in blood, plasma, and un-bound in interstitial fluid''. The VD of a drug represents the degree to which a drug is distributed in body tissue rather than the plasma. VD is directly proportional with the amount of drug distributed into tissue; a higher VD indicates a greater amount of tissue distribution. A VD greater than the total volume of body water (approximately 42 liters in humans) is possible, and would indicate that the drug is highly distributed into tissue. In other words, the volume of distribution is smaller in the drug staying in the plasma than that of a dr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bioequivalence
Bioequivalence is a term in pharmacokinetics used to assess the expected in vivo biological equivalence of two proprietary preparations of a drug. If two products are said to be bioequivalent it means that they would be expected to be, for all intents and purposes, the same. Birkett (2003) defined bioequivalence by stating that, "two pharmaceutical products are bioequivalent if they are pharmaceutically equivalent and their bioavailabilities (rate and extent of availability) after administration in the same molar dose are similar to such a degree that their effects, with respect to both efficacy and safety, can be expected to be essentially the same. Pharmaceutical equivalence implies the same amount of the same active substance(s), in the same dosage form, for the same route of administration and meeting the same or comparable standards." For The World Health Organization (WHO) "two pharmaceutical products are bioequivalent if they are pharmaceutically equivalent or pharmaceut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |