|
Bioequivalence
Bioequivalence is a term in pharmacokinetics used to assess the expected in vivo biological equivalence of two proprietary preparations of a drug. If two products are said to be bioequivalent it means that they would be expected to be, for all intents and purposes, the same. Birkett (2003) defined bioequivalence by stating that, "two pharmaceutical products are bioequivalent if they are pharmaceutically equivalent and their bioavailabilities (rate and extent of availability) after administration in the same molar dose are similar to such a degree that their effects, with respect to both efficacy and safety, can be expected to be essentially the same. Pharmaceutical equivalence implies the same amount of the same active substance(s), in the same dosage form, for the same route of administration and meeting the same or comparable standards." For The World Health Organization (WHO) "two pharmaceutical products are bioequivalent if they are pharmaceutically equivalent or pharmaceu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Generic Drug
A generic drug is a pharmaceutical drug that contains the same chemical substance as a drug that was originally protected by chemical patents. Generic drugs are allowed for sale after the patents on the original drugs expire. Because the active chemical substance is the same, the medical profile of generics is equivalent in performance. A generic drug has the same active pharmaceutical ingredient (API) as the original, but it may differ in some characteristics such as the manufacturing process, formulation, excipients, color, taste, and packaging. Although they may not be associated with a particular company, generic drugs are usually subject to government regulations in the countries in which they are dispensed. They are labeled with the name of the manufacturer and a generic non-proprietary name such as the United States Adopted Name (USAN) or International Nonproprietary Name (INN) of the drug. A generic drug must contain the same active ingredients as the original brand- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bioavailability
In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation. By definition, when a medication is administered intravenously, its bioavailability is 100%. However, when a medication is administered via routes other than intravenous, its bioavailability is generally lower than that of intravenous due to intestinal endothelium absorption and first-pass metabolism. Thereby, mathematically, bioavailability equals the ratio of comparing the area under the plasma drug concentration curve versus time (AUC) for the extravascular formulation to the AUC for the intravascular formulation. AUC is used because AUC is proportional to the dose that has entered the systemic circulation. Bioavailability of a drug is an average value; to take population variability into account, deviation range is shown as ±. To ensure that the drug taker who has poor absorption is dosed appropriately, the bottom value ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
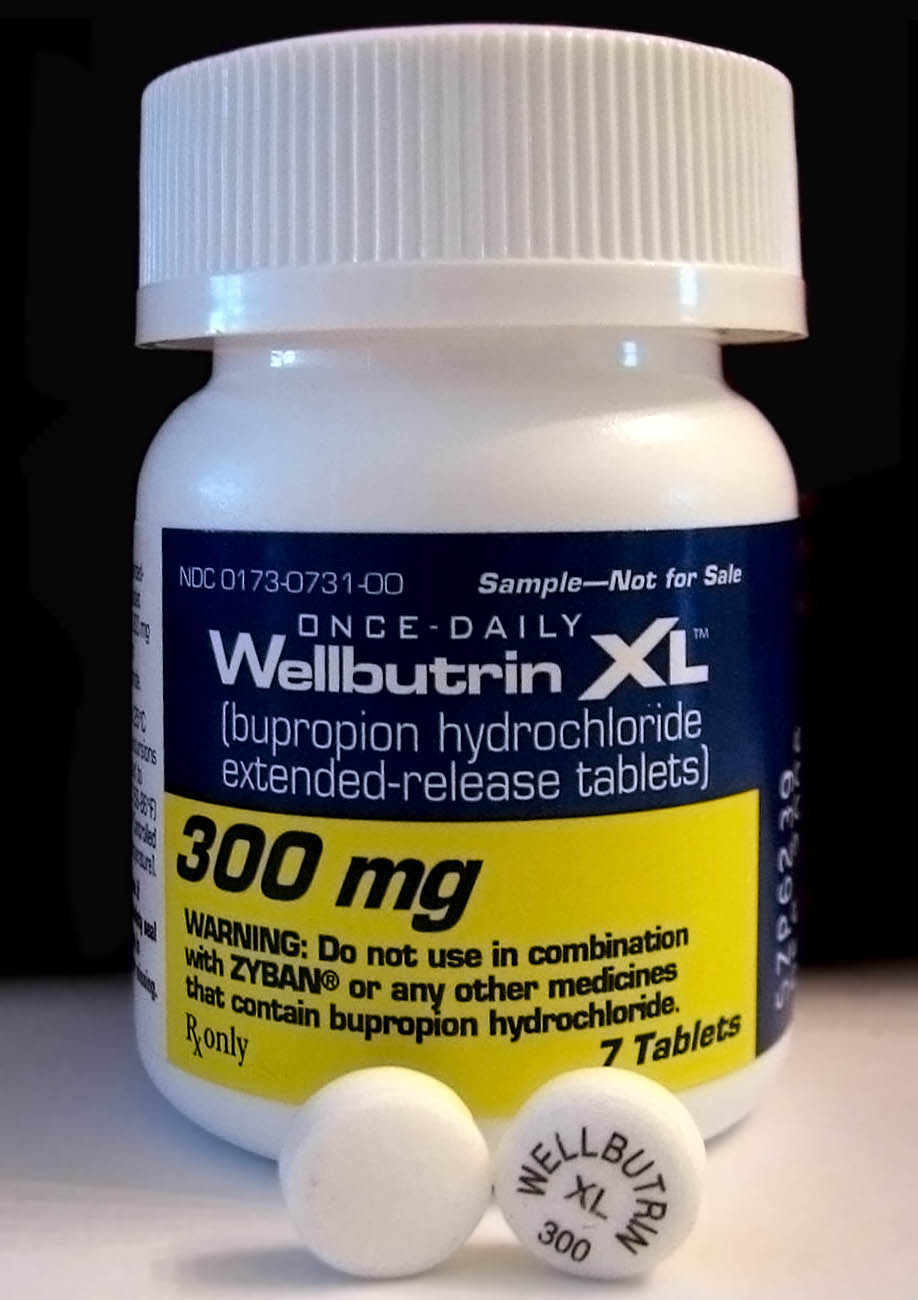
Bupropion Bioequivalency Comparison
Bupropion, sold under the brand names Wellbutrin and Zyban among others, is an atypical antidepressant primarily used to treat major depressive disorder and to support smoking cessation. It is also popular as an add-on medication in the cases of "incomplete response" to the first-line selective serotonin reuptake inhibitor (SSRI) antidepressant. Bupropion has several features that distinguish it from other antidepressants: it does not usually cause sexual dysfunction; it is not associated with weight gain and sleepiness, and it is more effective than SSRIs at improving symptoms of hypersomnia and fatigue. Bupropion does, however, carry a much higher risk of seizure than many other antidepressants and extreme caution must be taken in patients with a history of seizure disorder. Common adverse effects of bupropion with the greatest difference from placebo are dry mouth, nausea, constipation, insomnia, anxiety, tremor, and excessive sweating. Raised blood pressure is notable. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Abbreviated New Drug Application
An Abbreviated New Drug Application (ANDA) is an application for a U.S. generic drug approval for an existing licensed medication or approved drug. The ANDA is submitted to FDA's Center for Drug Evaluation and Research, Office of Generic Drugs, which provides for the review and ultimate approval of a generic drug product. Once approved, an applicant may manufacture and market the generic drug product to provide a safe, effective, low cost alternative to the American public. Electronic submissions of ANDAs have grown by 70% since November 2008. The Section IV challenge has been credited with suppressing new drug innovation. A generic drug product is one that is comparable to a patented drug product in dosage form, strength, route of administration, quality, performance characteristics and intended use. All approved products, both innovator and generic, are listed in FDA's Approved Drug Products with Therapeutic Equivalence Evaluations (Orange Book). Generic drug applications ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmacokinetics
Pharmacokinetics (from Ancient Greek ''pharmakon'' "drug" and ''kinetikos'' "moving, putting in motion"; see chemical kinetics), sometimes abbreviated as PK, is a branch of pharmacology dedicated to determining the fate of substances administered to a living organism. The substances of interest include any chemical xenobiotic such as: pharmaceutical drugs, pesticides, food additives, cosmetics, etc. It attempts to analyze chemical metabolism and to discover the fate of a chemical from the moment that it is administered up to the point at which it is completely eliminated from the body. Pharmacokinetics is the study of how an organism affects a drug, whereas pharmacodynamics (PD) is the study of how the drug affects the organism. Both together influence dosing, benefit, and adverse effects, as seen in PK/PD models. Overview Pharmacokinetics describes how the body affects a specific xenobiotic/chemical after administration through the mechanisms of absorption and distribut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, prescription and over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, animal foods & feed and veterinary products. The FDA's primary focus is enforcement of the Federal Food, Drug, and Cosmetic Act (FD&C), but the agency also enforces other laws, notably Section 361 of the Public Health Service Act, as well as associated regulations. Much of this regulatory-enforcement work is not directly related to food or drugs, but involves such things as regulating lasers, cellular phones, and condoms, as well as control of disease in contexts v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of medicinal products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).Set up by EC Regulation No. 2309/93 as the European Agency for the Evaluation of Medicinal Products, and renamed by EC Regulation No. 726/2004 to the European Medicines Agency, it had the acronym EMEA until December 2009. The European Medicines Agency does not call itself EMA either – it has no official acronym but may reconsider if EMA becomes commonly accepted (secommunication on new visual identity an). The EMA was set up in 1995, with funding from the European Union and the pharmaceutical industry, as well as indirect subsidy from member states, its stated intention to harmonise (but not replace) the work of existing national medicine regulatory bodies. The hope was that this plan would not o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Trial
Clinical trials are prospective biomedical or behavioral research studies on human subject research, human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, pharmaceutical drug, drugs, medical nutrition therapy, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received institutional review board, health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted. Depending on product type and development stage, investigators initially enroll volunteers or patients into small Pilot experiment, pi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ranbaxy
Ranbaxy Laboratories Limited was an Indian multinational pharmaceutical company that was incorporated in India in 1961 and remained an entity until 2014. The company went public in 1973. Ownership of Ranbaxy changed twice over the course of its history. In 2008, Japanese pharmaceutical company Daiichi Sankyo acquired a controlling share in Ranbaxy and in 2014, Sun Pharma acquired 100% of Ranbaxy in an all-stock deal. The Sun Pharma acquisition brought all new management to Ranbaxy, which had been laden with controversy (see Controversies below). Sun is the world's fifth largest specialty generic pharmaceutical company. History Formation Ranbaxy was started by Ranbir Singh and Gurbax Singh in 1937 as a distributor for Japanese company Shionogi. The name Ranbaxy blends the names of its founders: Ranbir and Gurbax. Bhai Mohan Singh bought the company in 1952 from his cousins Ranbir and Gurbax. After Bhai Mohan Singh's son Parvinder Singh joined the company in 1967, the company ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NBC News
NBC News is the news division of the American broadcast television network NBC. The division operates under NBCUniversal Television and Streaming, a division of NBCUniversal, which is, in turn, a subsidiary of Comcast. The news division's various operations report to the president of NBC News, Noah Oppenheim. The NBCUniversal News Group also comprises MSNBC, the network's 24-hour general news channel, business and consumer news channels CNBC and CNBC World, the Spanish language Noticias Telemundo and United Kingdom–based Sky News. NBC News aired the first regularly scheduled news program in American broadcast television history on February 21, 1940. The group's broadcasts are produced and aired from 30 Rockefeller Plaza, NBCUniversal's headquarters in New York City. The division presides over America's number-one-rated newscast, '' NBC Nightly News'', the world's first of its genre morning television program, '' Today'', and the longest-running television series in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ConsumerLab
ConsumerLab.com, LLC. is a privately held American company registered in White Plains, NY. It is a publisher of test results on health, wellness, and nutrition products. Consumer Labs is not a laboratory, but contracts studies to outside laboratories. It purchases supplement products and other consumer goods directly from public storefronts and publishes reports based on the results. It primarily derives revenue from the sale of subscriptions to its online publications. Other sources of revenue include a proprietary certification program, licensing fees, contents re-publication license fees and advertising. In 2000, ConsumerLab.com generated media attention when its testing of ginseng products revealed substantial pesticide contamination in many products. In 2008, they found 12 red yeast rice product samples to contain widely varying amounts of active ingredients and some included toxins. The testing was repeated in 2014 and 2018 with similar findings. In 2011, they found that tw ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)
.jpg)