|
Cis–trans Isomerism
''Cis''–''trans'' isomerism, also known as geometric isomerism or configurational isomerism, is a term used in chemistry that concerns the spatial arrangement of atoms within molecules. The prefixes "''cis''" and "''trans''" are from Latin: "this side of" and "the other side of", respectively. In the context of chemistry, ''cis'' indicates that the functional groups (substituents) are on the same side of some plane, while ''trans'' conveys that they are on opposing (transverse) sides. ''Cis''–''trans'' isomers are stereoisomers, that is, pairs of molecules which have the same formula but whose functional groups are in different orientations in three-dimensional space. ''Cis-trans'' notation does not always correspond to ''E''–''Z'' isomerism, which is an ''absolute'' stereochemical description. In general, ''cis''–''trans'' stereoisomers contain double bonds that do not rotate, or they may contain ring structures, where the rotation of bonds is restricted or prevented ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cis-2-butene
But-2-ene is an acyclic alkene with four carbon atoms. It is the simplest alkene exhibiting ''cis''/''trans''-isomerism (also known as (''E''/''Z'')-isomerism); that is, it exists as two geometric isomers ''cis''-but-2-ene ((''Z'')-but-2-ene) and ''trans-''but-2-ene ((''E'')-but-2-ene). It is a petrochemical, produced by the catalytic cracking of crude oil or the dimerization of ethylene. Its main uses are in the production of gasoline (petrol) and butadiene,. although some but-2-ene is also used to produce the solvent butanone via hydration to 2-butanol followed by oxidation. The two isomers are extremely difficult to separate by distillation because of the proximity of their boiling points (~4 °C for ''cis'' and ~1 °C for ''trans'' [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
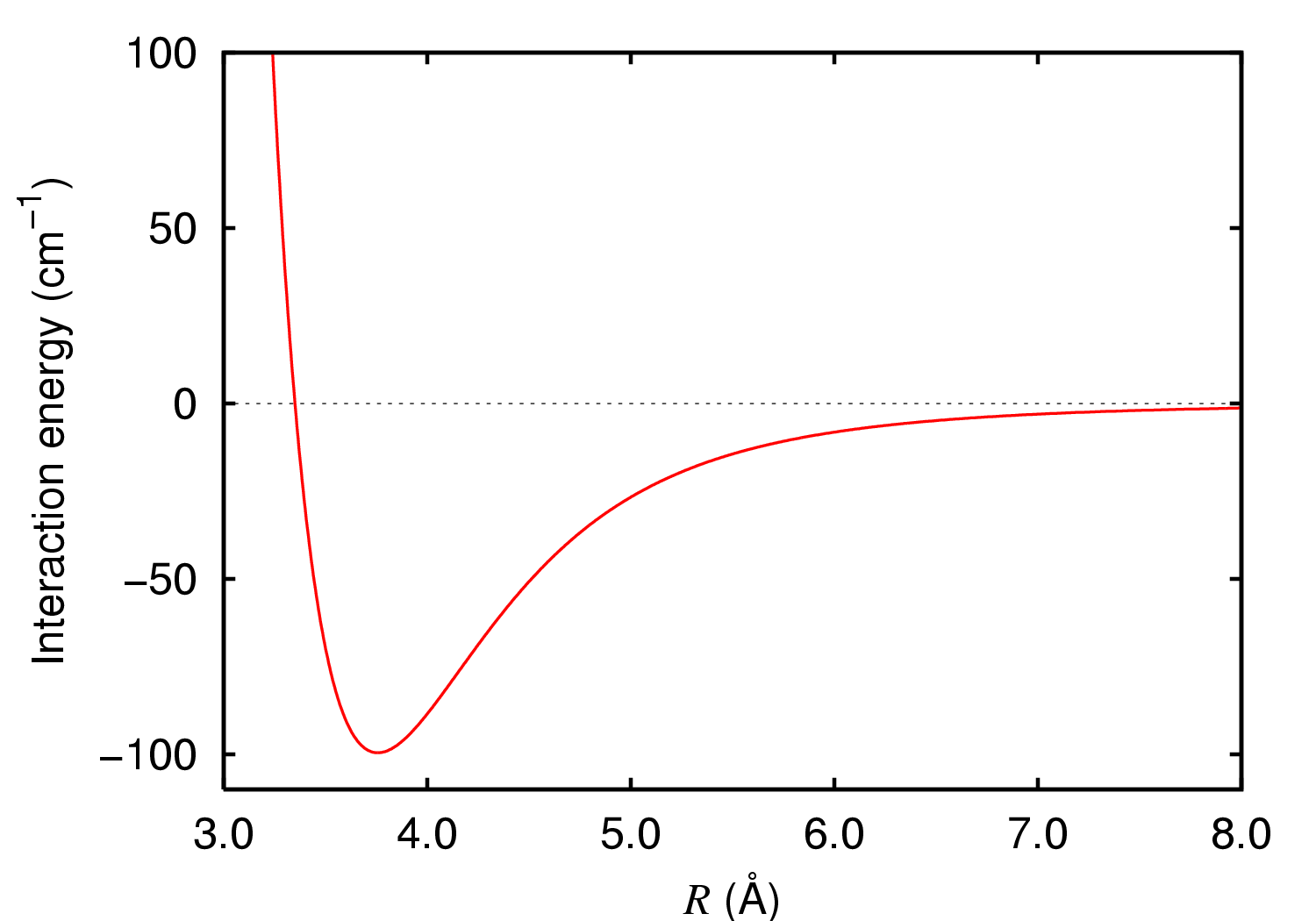
London Dispersion Forces
London dispersion forces (LDF, also known as dispersion forces, London forces, instantaneous dipole–induced dipole forces, fluctuating induced dipole bonds or loosely as van der Waals forces) are a type of intermolecular force acting between atoms and molecules that are normally electrically symmetric; that is, the electrons are symmetrically distributed with respect to the nucleus. They are part of the van der Waals forces. The LDF is named after the German physicist Fritz London. They are the weakest intermolecular force. Introduction The electron distribution around an atom or molecule undergoes fluctuations in time. These fluctuations create instantaneous electric fields which are felt by other nearby atoms and molecules, which in turn adjust the spatial distribution of their own electrons. The net effect is that the fluctuations in electron positions in one atom induce a corresponding redistribution of electrons in other atoms, such that the electron motions become corre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intermolecular Forces
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction or repulsion which act between atoms and other types of neighbouring particles, e.g. atoms or ions. Intermolecular forces are weak relative to intramolecular forces – the forces which hold a molecule together. For example, the covalent bond, involving sharing electron pairs between atoms, is much stronger than the forces present between neighboring molecules. Both sets of forces are essential parts of force fields frequently used in molecular mechanics. The investigation of intermolecular forces starts from macroscopic observations which indicate the existence and action of forces at a molecular level. These observations include non-ideal-gas thermodynamic behavior reflected by virial coefficients, vapor pressure, viscosity, superficial tension, and absorption data. The first reference to the nature of microscopic fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bond Dipole Moment
In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end. Polar molecules must contain one or more polar bonds due to a difference in electronegativity between the bonded atoms. Molecules containing polar bonds have no molecular polarity if the bond dipoles cancel each other out by symmetry. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points. Polarity of bonds Not all atoms attract electrons with the same force. The amount of "pull" an atom exerts on its electrons is called its electronegativity. Atoms with high electronegativitiessuch as fluorine, oxygen, and nitrogenexert a greater pull on electrons than atoms with lower electronegativities such as alkali metals and alkaline ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-dichloroethene
1,2-Dichloroethene, commonly called 1,2-dichloroethylene or 1,2-DCE, is the name for a pair of organochlorine compounds with the molecular formula CHCl. They are both colorless liquids with a sweet odor. It can exist as either of two geometric isomers, ''cis''-1,2-dichloroethene or ''trans''-1,2-dichloroethene, but is often used as a mixture of the two. They have modest solubility in water. These compounds have some applications as a degreasing solvent. In contrast to most ''cis-trans'' compounds, the ''Z'' isomer (''cis'') is more stable than the ''E'' isomer (''trans'') by 0.4 kcal/mol. Production and use ''cis''-DCE, the ''Z'' isomer, is obtainable by the controlled chlorination of acetylene: :CH + Cl → CHCl Industrially both isomers arise as byproducts of the production of vinyl chloride, which is produced on a vast scale. Unlike vinyl chloride, the 1,2-dichloroethylene isomers do not polymerize. ''trans''-DCE has applications including electronics cleaning, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentene
Pentenes are alkenes with the chemical formula . Each contains one double bond within its molecular structure. Six different compounds are in this class, differing from each other by whether the carbon atoms are attached linearly or in a branched structure, and whether the double bond has a ''cis'' or ''trans'' form. Straight-chain isomers 1-Pentene is an alpha-olefin. Most often, 1-pentene is made as a byproduct of catalytic or thermal cracking of petroleum, or during production of ethylene and propylene via thermal cracking of hydrocarbon fractions. The only commercial manufacturer of 1-pentene is Sasol Ltd, where it is separated from crude made by the Fischer–Tropsch process. 2-Pentene has two geometric isomers, ''cis''-2-pentene and ''trans''-2-pentene. ''Cis''-2-Pentene is used in olefin metathesis. Branched-chain isomers The branched isomers are 2-methylbut-1-ene, 3-methylbut-1-ene (isopentene), and 2-methylbut-2-ene (isoamylene). Isoamylene is one of three main ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trans-1,2-dichloroethene
1,2-Dichloroethene, commonly called 1,2-dichloroethylene or 1,2-DCE, is the name for a pair of organochlorine compounds with the molecular formula CHCl. They are both colorless liquids with a sweet odor. It can exist as either of two geometric isomers, ''cis''-1,2-dichloroethene or ''trans''-1,2-dichloroethene, but is often used as a mixture of the two. They have modest solubility in water. These compounds have some applications as a degreasing solvent. In contrast to most ''cis-trans'' compounds, the ''Z'' isomer (''cis'') is more stable than the ''E'' isomer (''trans'') by 0.4 kcal/mol. Production and use ''cis''-DCE, the ''Z'' isomer, is obtainable by the controlled chlorination of acetylene: :CH + Cl → CHCl Industrially both isomers arise as byproducts of the production of vinyl chloride, which is produced on a vast scale. Unlike vinyl chloride, the 1,2-dichloroethylene isomers do not polymerize. ''trans''-DCE has applications including electronics cleaning, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cis-1,2-dichloroethene
1,2-Dichloroethene, commonly called 1,2-dichloroethylene or 1,2-DCE, is the name for a pair of organochlorine compounds with the molecular formula CHCl. They are both colorless liquids with a sweet odor. It can exist as either of two geometric isomers, ''cis''-1,2-dichloroethene or ''trans''-1,2-dichloroethene, but is often used as a mixture of the two. They have modest solubility in water. These compounds have some applications as a degreasing solvent. In contrast to most ''cis-trans'' compounds, the ''Z'' isomer (''cis'') is more stable than the ''E'' isomer (''trans'') by 0.4 kcal/mol. Production and use ''cis''-DCE, the ''Z'' isomer, is obtainable by the controlled chlorination of acetylene: :CH + Cl → CHCl Industrially both isomers arise as byproducts of the production of vinyl chloride, which is produced on a vast scale. Unlike vinyl chloride, the 1,2-dichloroethylene isomers do not polymerize. ''trans''-DCE has applications including electronics cleaning, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trans-2-pentene
Trans- is a Latin prefix meaning "across", "beyond", or "on the other side of". Used alone, trans may refer to: Arts, entertainment, and media * Trans (festival), a former festival in Belfast, Northern Ireland, United Kingdom * ''Trans'' (film), a 1998 American film * Trans Corp, an Indonesian business unit of CT Corp in the fields of media, lifestyle, and entertainment ** Trans Media, a media subsidiary of Trans Corp *** Trans TV, an Indonesian television network *** Trans7, an Indonesian television network Literature * '' Trans: Gender and Race in an Age of Unsettled Identities'', a 2016 book by Rogers Brubaker * '' Trans: When Ideology Meets Reality'', a 2021 book by Helen Joyce Music * ''Trans'' (album), by Neil Young * ''Trans'' (Stockhausen), a 1971 orchestral composition Places * Trans, Mayenne, France, a commune * Trans, Switzerland, a village Science and technology * Trans effect in inorganic chemistry, the increased lability of ligands that are trans to certain ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cis-2-pentene
Cis or cis- may refer to: Places * Cis, Trentino, in Italy * In Poland: ** Cis, Świętokrzyskie Voivodeship, south-central ** Cis, Warmian-Masurian Voivodeship, north Math, science and biology * cis (mathematics) (cis(''θ'')), a trigonometric mathematical function related to Euler's formula * ''Cis'' (beetle), genus * Cis–trans isomerism, in chemistry * cis-regulatory element, regions of non-coding DNA which regulate the transcription of nearby genes Other uses * Cisgender, in contrast with transgender * C♯ (musical note), known as cis See also * CIS (other) * * Ciss (other) Ciss (pronounced SIHS) is a Senegalese surname. Notable people with the surname include: * Amadou Ciss (born 1999), Senegalese footballer who plays for Fortuna Sittard *Elhadji Ciss Abdoulaye Elhadji Ciss (born 26 June 1994) is a Senegalese p ... * Csi (other) {{disambiguation, geo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |