|
Chemotronics
{{Short description, Intersection field of Chemistry and Electronics Chemotronics is an intersection field of chemistry (especially electrochemistry) and electronics dealing with the design of electrochemical and optical chemical sensors. One of pioneers of this field was Alexander Frumkin. See also *Amperostat A galvanostat (also known as amperostat) is a control and measuring device capable of keeping the current through an electrolytic cell in coulometric titrations constant, disregarding changes in the load itself.
Its main feature is its ''nearly' ...
* [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alexander Frumkin
Alexander Naumovich Frumkin (Алекса́ндр Нау́мович Фру́мкин) (October 24, 1895 – May 27, 1976) was a Russian/Soviet electrochemist, member of the Russian Academy of Sciences since 1932, founder of the Russian Journal of Electrochemistry '' Elektrokhimiya'' and receiver of the Hero of Socialist Labor award. The Russian Academy of Sciences' A.N. Frumkin Institute of Physical Chemistry and Electrochemistry is named after him. Biography Early life Frumkin was born in Kishinev, in the Bessarabia Governorate of the Russian Empire (present-day Moldova) to a Jewish family; his father was an insurance salesman. His family moved to Odessa, where he received his primary schooling; he continued his education in Strasbourg, and then at the University of Bern. Frumkin's first published articles appeared in 1914, when he was only 19; in 1915, he received his first degree, back in Odessa. Two years later, the seminal article "Electrocapillary Phenomena and El ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outcome of a particular chemical change, or vice versa. These reactions involve electrons moving via an electronically-conducting phase (typically an external electrical circuit, but not necessarily, as in electroless plating) between electrodes separated by an ionically conducting and electronically insulating electrolyte (or ionic species in a solution). When a chemical reaction is driven by an electrical potential difference, as in electrolysis, or if a potential difference results from a chemical reaction as in an electric battery or fuel cell, it is called an ''electrochemical'' reaction. Unlike in other chemical reactions, in electrochemical reactions electrons are not transferred directly between atoms, ions, or molecules, but via the af ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronics
The field of electronics is a branch of physics and electrical engineering that deals with the emission, behaviour and effects of electrons using electronic devices. Electronics uses active devices to control electron flow by amplification and rectification, which distinguishes it from classical electrical engineering, which only uses passive effects such as resistance, capacitance and inductance to control electric current flow. Electronics has hugely influenced the development of modern society. The central driving force behind the entire electronics industry is the semiconductor industry sector, which has annual sales of over $481 billion as of 2018. The largest industry sector is e-commerce, which generated over $29 trillion in 2017. History and development Electronics has hugely influenced the development of modern society. The identification of the electron in 1897, along with the subsequent invention of the vacuum tube which could amplify and rectify small ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amperostat
A galvanostat (also known as amperostat) is a control and measuring device capable of keeping the current through an electrolytic cell in coulometric titrations constant, disregarding changes in the load itself. Its main feature is its ''nearly'' "''infinite''" (i.e. extremely high in respect to common loads) internal resistance. The designation "''galvanostat''" is mainly used in electrochemistry: this device differs from common constant current sources by its ability to supply ''and measure'' a wide range of currents (from picoamperes to amperes) of both polarities. The galvanostat responds to changes in the resistance of the cell by varying its output potential: as Ohm's law shows, : = the variable system resistance and the controlled voltage are directly proportional, i.e. : U_c = where *''I_o'' is the electric current that is kept constant *''U_c'' is the output control voltage of the amperostat *''R_v'' is the electrical resistance that varies; thus, an increase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bioelectrochemistry
Bioelectrochemistry is a branch of electrochemistry and biophysical chemistry concerned with electrophysiological topics like cell electron-proton transport, cell membrane potentials and electrode reactions of redox enzymes. History The beginnings of bioelectrochemistry, as well as those of electrochemistry, are closely related to physiology through the works of Luigi Galvani and then Alessandro Volta. The first modern work in this field is considered that of the German physiologist Julius Bernstein (1902) concerning the source of biopotentials due to different ion concentration through the cell's membrane. The domain of bioelectrochemistry has grown considerably over the past century, maintaining the close connections to various medical and biological and engineering disciplines like electrophysiology, biomedical engineering, and enzyme kinetics. The achievements in this field have been awarded several Nobel prizes for Physiology or Medicine. Among prominent electrochemists who ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bioelectronics
Bioelectronics is a field of research in the convergence of biology and electronics. Definitions At the first C.E.C. Workshop, in Brussels in November 1991, bioelectronics was defined as 'the use of biological materials and biological architectures for information processing systems and new devices'. Bioelectronics, specifically bio-molecular electronics, were described as 'the research and development of bio-inspired (i.e. self-assembly) inorganic and organic materials and of bio-inspired (i.e. massive parallelism) hardware architectures for the implementation of new information processing systems, sensors and actuators, and for molecular manufacturing down to the atomic scale'. The National Institute of Standards and Technology (NIST), an agency of the United States Department of Commerce, defined bioelectronics in a 2009 report as "the discipline resulting from the convergence of biology and electronics". Sources for information about the field include the Institute of Electr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemical Engineering
Electrochemical engineering is the branch of chemical engineering dealing with the technological applications of electrochemical phenomena, such as electrosynthesis of chemicals, electrowinning and refining of metals, flow batteries and fuel cells, surface modification by electrodeposition, electrochemical separations and corrosion. This discipline is an overlap between electrochemistry and chemical engineering. According with the IUPAC, the term ''electrochemical engineering'' is reserved for electricity intensive processes for industrial or energy storage applications, and should not be confused with ''applied electrochemistry'', which comprises small batteries, amperometric sensors, microfluidic devices, microelectrodes, solid-state devices, voltammetry at disc electrodes, etc. More than 6% of the electricity is consumed by large-scale electrochemical operations in the US. Scope Electrochemical engineering combines the study of heterogeneous charge transfer at electrode/ele ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
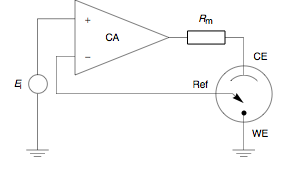
Potentiostat
A potentiostat is the electronic hardware required to control a three electrode cell and run most electroanalytical experiments. A ''Bipotentiostat'' and ''polypotentiostat'' are potentiostats capable of controlling two working electrodes and more than two working electrodes, respectively. The system functions by maintaining the potential of the working electrode at a constant level with respect to the reference electrode by adjusting the current at an auxiliary electrode. The heart of the different potentiostatic electronic circuits is an operational amplifier (op amp). It consists of an electric circuit which is usually described in terms of simple op amps. Primary use This equipment is fundamental to modern electrochemical studies using three electrode systems for investigations of reaction mechanisms related to redox chemistry and other chemical phenomena. The dimensions of the resulting data depend on the experiment. In voltammetry, electric current in amps is pl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analytical Chemistry
Analytical chemistry studies and uses instruments and methods to separate, identify, and quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separation isolates analytes. Qualitative analysis identifies analytes, while quantitative analysis determines the numerical amount or concentration. Analytical chemistry consists of classical, wet chemical methods and modern, instrumental methods. Classical qualitative methods use separations such as precipitation, extraction, and distillation. Identification may be based on differences in color, odor, melting point, boiling point, solubility, radioactivity or reactivity. Classical quantitative analysis uses mass or volume changes to quantify amount. Instrumental methods may be used to separate samples using chromatography, electrophoresis or field flow fractionation. Then qualitative and quantitative analysis can be performed, often with t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)