|
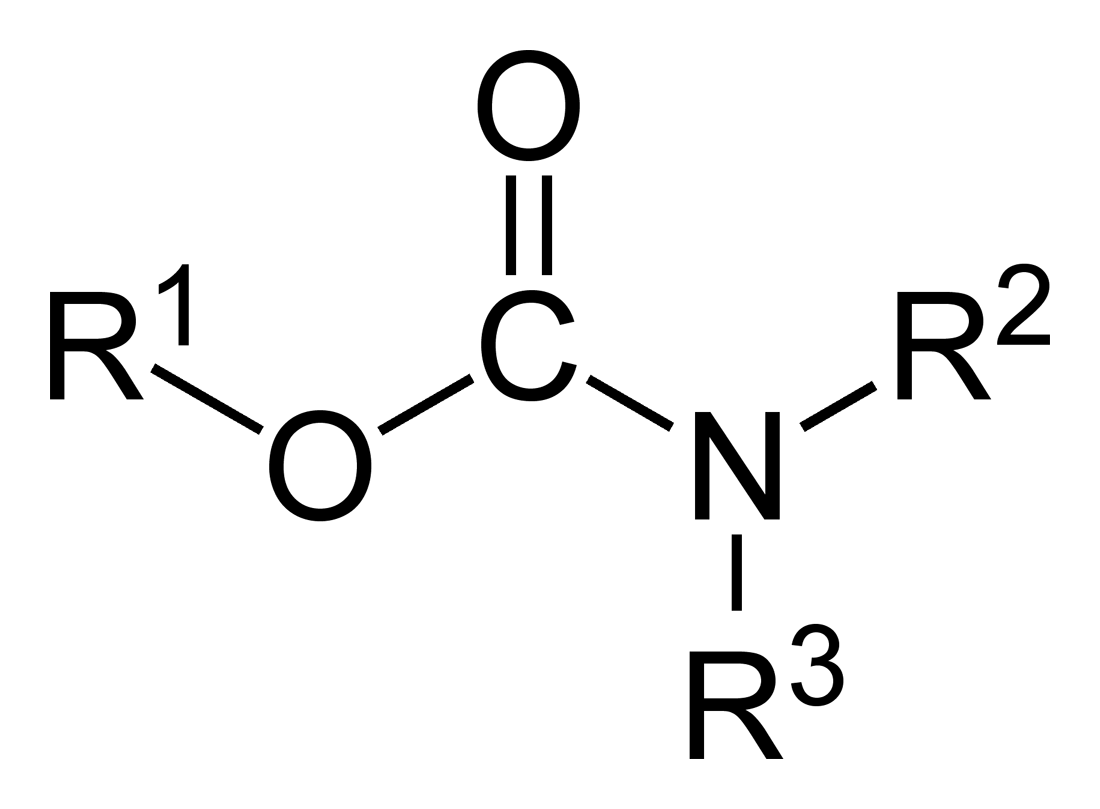
Carbamate
In organic chemistry, a carbamate is a category of organic compounds with the general formula and structure , which are formally derived from carbamic acid (). The term includes organic compounds (e.g., the ester ethyl carbamate), formally obtained by replacing one or more of the hydrogen atoms by other organic functional groups; as well as salts with the carbamate anion (e.g. ammonium carbamate). Polymers whose units are joined by carbamate groups are an important family of plastics, the polyurethanes. Properties While carbamic acids are unstable, many carbamate esters or ionic) are stable and well known. Equilibrium with carbonate and bicarbonate In water solutions, the carbamate anion slowly equilibrates with the ammonium cation and the carbonate or bicarbonate anions: : : Calcium carbamate is soluble in water, whereas calcium carbonate is not. Adding a calcium salt to an ammonium carbamate/carbonate solution will precipitate some calcium carbonat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Carbamate
Ethyl carbamate (also called urethane) is an organic compound with the formula CH3CH2OC(O)NH2. It is an ester of carbamic acid and a white solid. Despite its name, it is not a component of polyurethanes. Because it is a carcinogen, it is rarely used, but naturally forms in low quantities in many types of fermented foods and drinks. Synthesis It is produced industrially by heating urea and Ethanol, ethyl alcohol. It arise also by the action of ammonia on ethyl chloroformate. Uses Biomedical applications Ethyl carbamate has been used as an antineoplastic agent and for other medicinal purposes, but this application ended after it was discovered to be carcinogenic in 1943. However, Japanese usage in medical injections continued and from 1950 to 1975 an estimated 100 million 2 ml ampules of 7-to-15% solutions of ethyl carbamate were injected into patients as a co-solvent in water for dissolving water-insoluble analgesics used for post-operation pain. These doses were estim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbamic Acid
Carbamic acid, which might also be called aminoformic acid or aminocarboxylic acid, is the chemical compound with the formula . It can be obtained by the reaction of ammonia and carbon dioxide at very low temperatures, which also yields an equal amount of ammonium carbamate . The compound is stable only up to about 250 K (−23 °C); at higher temperatures it decomposes into those two gases. The solid apparently consists of dimers, with the two molecules connected by hydrogen bonds between the two carboxyl groups –COOH.J. B. Bossa, P. Theulé, F. Duvernay, F. Borget and T. Chiavassa (2008): "Carbamic acid and carbamate formation in NH3:CO2 ices – UV irradiation versus thermal processes". ''Astronomy and Astrophysics'', volume 492, issue 3, pages 719-724. Carbamic acid could be seen as both an amine and carboxylic acid, and therefore an amino acid;R. K. Khanna and M. H. Moore (1999): "Carbamic acid: molecular structure and IR spectra". ''Spectrochimica Acta P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Carbamate
Ammonium carbamate is a chemical compound with the formula consisting of ammonium cation and carbamate anion . It is a white solid that is extremely soluble in water, less so in alcohol. Ammonium carbamate can be formed by the reaction of ammonia with carbon dioxide , and will slowly decompose to those gases at ordinary temperatures and pressures. It is an intermediate in the industrial synthesis of urea , an important fertilizer. Properties Solid-gas equilibrium In a closed container solid ammonium carbamate is in equilibrium with carbon dioxide and ammonia R. N. Bennett, P. D. Ritchie, D. Roxburgh and J. Thomson (1953): "The system ammonia + carbon dioxide + ammonium carbamate. Part I. — The equilibrium of thermal dissociation of ammonium carbamate". ''Transactions of the Faraday Society'', volume 49, pages 925-929. : Lower temperatures shift the equilibrium towards the carbamate. At higher temperatures ammonium carbamate condenses into urea: : This reaction was f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their nonpolar core of carbon atoms and thus add chemical character to carbon chains. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
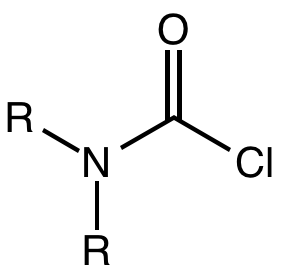
Carbamoyl Chloride
A carbamoyl chloride is the functional group with the formula R2NC(O)Cl. The parent carbamoyl chloride, H2NCOCl is unstable, but many N-substituted analogues are known. Most examples are moisture sensitive, colourless, and soluble in nonpolar organic solvents. An example is dimethylcarbamoyl chloride (m.p. −90 °C and b.p. 93 °C). Carbamoyl chlorides are used to prepare a number of pesticides, e.g. carbofuran and aldicarb. Production and examples Carbamoyl chlorides are prepared by the reaction of an amine with phosgene: :2 R2NH + COCl2 → R2NCOCl + 2NH2l They also arise by the addition of hydrogen chloride to isocyanates: :RNCO + HCl → RNHCOCl In this way, carbamonyl chlorides can be prepared with N-H functionality. Reactions In a reaction that is typically avoided, hydrolysis of carbamoyl chlorides gives carbamic acids: :R2NCOCl + H2O → R2NC(O)OH + HCl Owing to the influence of the amino group, these compounds are less hydrolytically sensitive than the usual acid chl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. It is a trace gas in Earth's atmosphere at 421 parts per million (ppm), or about 0.04% by volume (as of May 2022), having risen from pre-industrial levels of 280 ppm. Burning fossil fuels is the primary cause of these increased CO2 concentrations and also the primary cause of climate change.IPCC (2022Summary for policy makersiClimate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA Carbon dioxide is soluble in water and is found in groundwater, lakes, i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroformate
Chloroformates are a class of organic compounds with the formula ROC(O)Cl. They are formally esters of chloroformic acid. Most are colorless, volatile liquids that degrade in moist air. A simple example is methyl chloroformate, which is commercially available. Chloroformates are used as reagents in organic chemistry. For example, benzyl chloroformate is used to introduce the Cbz (carboxybenzyl) protecting group and fluorenylmethyloxycarbonyl chloride is used to introduce the FMOC protecting group. Chloroformates are popular in the field of chromatography as derivatization agents. They convert polar compounds into less polar more volatile derivatives. In this way, chloroformates enable relatively simple transformation of large array of metabolites (aminoacids, amines, carboxylic acids, phenols) for analysis by gas chromatography / mass spectrometry. Reactions The reactivity of chloroformates and acyl chlorides are similar. Representative reactions are: * Reaction with amine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substitue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Curtius Rearrangement
The Curtius rearrangement (or Curtius reaction or Curtius degradation), first defined by Theodor Curtius in 1885, is the thermal decomposition of an acyl azide to an isocyanate with loss of nitrogen gas. The isocyanate then undergoes attack by a variety of nucleophiles such as water, alcohols and amines, to yield a primary amine, carbamate or urea derivative respectively. Several reviews have been published. Preparation of acyl azide The acyl azide is usually made from the reaction of acid chlorides or anydrides with sodium azide or trimethylsilyl azide. Acyl azides are also obtained from treating acylhydrazines with nitrous acid. Alternatively, the acyl azide can be formed by the direct reaction of a carboxylic acid with diphenylphosphoryl azide (DPPA). Reaction mechanism It was believed that the Curtius rearrangement was a two-step processes, with the loss of nitrogen gas forming an acyl nitrene, followed by migration of the R-group to give the isocyanate. However, re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
-3D-balls.png)

