|
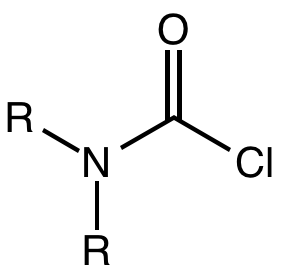
Carbamoyl Chloride
A carbamoyl chloride is the functional group with the formula R2NC(O)Cl. The parent carbamoyl chloride, H2NCOCl is unstable, but many N-substituted analogues are known. Most examples are moisture sensitive, colourless, and soluble in nonpolar organic solvents. An example is dimethylcarbamoyl chloride (m.p. −90 °C and b.p. 93 °C). Carbamoyl chlorides are used to prepare a number of pesticides, e.g. carbofuran and aldicarb. Production and examples Carbamoyl chlorides are prepared by the reaction of an amine with phosgene: :2 R2NH + COCl2 → R2NCOCl + 2NH2l They also arise by the addition of hydrogen chloride to isocyanates: :RNCO + HCl → RNHCOCl In this way, carbamonyl chlorides can be prepared with N-H functionality. Reactions In a reaction that is typically avoided, hydrolysis of carbamoyl chlorides gives carbamic acids: :R2NCOCl + H2O → R2NC(O)OH + HCl Owing to the influence of the amino group, these compounds are less hydrolytically sensitive than the usual acid chlo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their nonpolar core of carbon atoms and thus add chemical character to carbon chains. Fun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylcarbamoyl Chloride
Dimethylcarbamoyl chloride (DMCC) is a reagent for transferring a dimethylcarbamoyl group to alcoholic or phenolic hydroxyl groups forming dimethyl carbamates, usually having pharmacological or pesticidal activities. Because of its high toxicity and its carcinogenic properties shown in animal experiments and presumably also in humans, dimethylcarbamoyl chloride can only be used under stringent safety precautions. Production and occurrence The production of dimethylcarbamoyl chloride from phosgene and dimethylamine was reported as early as 1879 (reported as "Dimethylharnstoffchlorid" – dimethylurea chloride). : DMCC can be produced in high yields (90%) at 275 °C by reacting phosgene with gaseous dimethylamine in a flow reactor. To suppress the formation of ureas, excess phosgene is used (in a 3:1 ratio). The reaction can also be carried out at the laboratory scale with diphosgene or triphosgene and an aqueous dimethylamine solution in the two-phase system of benzene–xy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbofuran
Carbofuran is a carbamate pesticide, widely used around the world to control insects on a wide variety of field crops, including potatoes, corn and soybeans. It is a systemic insecticide, which means that the plant absorbs it through the roots, and from there the plant distributes it throughout its organs where insecticidal concentrations are attained. Carbofuran also has contact activity against pests. It is one of the most toxic pesticides still in use. It is marketed under the trade names Furadan, by FMC Corporation and Curaterr 10 GR, by Bayer among several others. Carbofuran exhibits toxicity mediated by the same mechanism as that of the notorious V-series nerve agents and presents a risk to human health. It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the United States Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities which produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldicarb
Aldicarb is a carbamate insecticide which is the active substance in the pesticide Temik. It is effective against thrips, aphids, spider mites, lygus, fleahoppers, and leafminers, but is primarily used as a nematicide. Aldicarb is a cholinesterase inhibitor which prevents the breakdown of acetylcholine in the synapse. In case of severe poisoning, the victim dies of respiratory failure. Aldicarb was first synthesized in 1965 by Payne and Weiden, and was sold on the market for the first time in 1970. The synthesis of aldicarb results in both the E and Z isomers. Aldicarb is one of the most widely used pesticides internationally, and is also one of the most environmentally toxic. Aldicarb poisoning from agricultural water runoff has led to the destruction of healthy ecosystems and the irreversible poisoning of fertile agricultural land. Aldicarb is effective where resistance to organophosphate insecticides has developed, and is extremely important in potato production, where ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride gas and hydrochloric acid are important in technology and industry. Hydrochloric acid, the aqueous solution of hydrogen chloride, is also commonly given the formula HCl. Reactions Hydrogen chloride is a diatomic molecule, consisting of a hydrogen atom H and a chlorine atom Cl connected by a polar covalent bond. The chlorine atom is much more electronegative than the hydrogen atom, which makes this bond polar. Consequently, the molecule has a large dipole moment with a negative partial charge (δ−) at the chlorine atom and a positive partial charge (δ+) at the hydrogen atom. In part because of its high polarity, HCl is very soluble in water (and in other polar solvents). Upon contact, and HCl combine to form hydronium cations and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isocyanate
In organic chemistry, isocyanate is the functional group with the formula . Organic compounds that contain an isocyanate group are referred to as isocyanates. An organic compound with two isocyanate groups is known as a diisocyanate. Diisocyanates are manufactured for the production of polyurethanes, a class of polymers. Isocyanates should not be confused with cyanate esters and isocyanides, very different families of compounds. The cyanate (cyanate ester) functional group () is arranged differently from the isocyanate group (). Isocyanides have the connectivity , lacking the oxygen of the cyanate groups. Structure and bonding In terms of bonding, isocyanates are closely related to carbon dioxide (CO2) and carbodiimides (C(NR)2). The C−N=C=O unit that defines isocyanates is planar, and the N=C=O linkage is nearly linear. In phenyl isocyanate, the C=N and C=O distances are respectively 1.195 and 1.173 Å. The C-N=C angle is 134.9° and the N=C=O angle is 173.1°. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbamic Acid
Carbamic acid, which might also be called aminoformic acid or aminocarboxylic acid, is the chemical compound with the formula . It can be obtained by the reaction of ammonia and carbon dioxide at very low temperatures, which also yields an equal amount of ammonium carbamate . The compound is stable only up to about 250 K (−23 °C); at higher temperatures it decomposes into those two gases. The solid apparently consists of dimers, with the two molecules connected by hydrogen bonds between the two carboxyl groups –COOH.J. B. Bossa, P. Theulé, F. Duvernay, F. Borget and T. Chiavassa (2008): "Carbamic acid and carbamate formation in NH3:CO2 ices – UV irradiation versus thermal processes". ''Astronomy and Astrophysics'', volume 492, issue 3, pages 719-724. Carbamic acid could be seen as both an amine and carboxylic acid, and therefore an amino acid;R. K. Khanna and M. H. Moore (1999): "Carbamic acid: molecular structure and IR spectra". ''Spectrochimica Acta Part A: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid Chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example of an acyl chloride is acetyl chloride, . Acyl chlorides are the most important subset of acyl halides. Nomenclature Where the acyl chloride moiety takes priority, acyl chlorides are named by taking the name of the parent carboxylic acid, and substituting ''-yl chloride'' for ''-ic acid''. Thus: : : When other functional groups take priority, acyl chlorides are considered prefixes — ''chlorocarbonyl-'': : Properties Lacking the ability to form hydrogen bonds, acyl chlorides have lower boiling and melting points than similar carboxylic acids. For example, acetic acid boils at 118 °C, whereas acetyl chloride boils at 51 °C. Like most carbonyl compounds, infrared spectroscopy reveals a band near 1750 cm−1. The simplest s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |