|
Calcium Bicarbonate
Calcium bicarbonate, also called calcium hydrogencarbonate, has the chemical formula Ca(HCO3)2. The term does not refer to a known solid compound; it exists only in aqueous solution containing calcium (Ca2+), bicarbonate (), and carbonate () ions, together with dissolved carbon dioxide (CO2). The relative concentrations of these carbon-containing species depend on the pH; bicarbonate predominates within the range 6.36–10.25 in fresh water. All waters in contact with the atmosphere absorb carbon dioxide, and as these waters come into contact with rocks and sediments they acquire metal ions, most commonly calcium and magnesium, so most natural waters that come from streams, lakes, and especially wells, can be regarded as dilute solutions of these bicarbonates. These hard waters tend to form carbonate scale in pipes and boilers and they react with soaps to form an undesirable scum. Attempts to prepare compounds such as solid calcium bicarbonate by evaporating its solution to dr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Bicarbonate
Magnesium bicarbonate or magnesium hydrogencarbonate, Mg( H CO3)2, is the bicarbonate salt of magnesium. It can be formed through the reaction of dilute solutions of carbonic acid (such as seltzer water) and magnesium hydroxide (milk of magnesia). It can be prepared through the synthesis of magnesium acetate and sodium bicarbonate: :: Magnesium bicarbonate exists only in aqueous solution. Magnesium does not form solid bicarbonate as does lithium. To produce it, a suspension of magnesium hydroxide is treated with pressurized carbon dioxide, producing a solution of magnesium bicarbonate: ::Mg(OH)2 + 2 CO2 → Mg(HCO3)2 Drying the resulting solution causes the magnesium bicarbonate to decompose Decomposition or rot is the process by which dead organic substances are broken down into simpler organic or inorganic matter such as carbon dioxide, water, simple sugars and mineral salts. The process is a part of the nutrient cycle and is ..., yielding magnesium carbonate, ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Bicarbonate
Ammonium bicarbonate is an inorganic compound with formula (NH4)HCO3. The compound has many names, reflecting its long history. Chemically speaking, it is the bicarbonate salt of the ammonium ion. It is a colourless solid that degrades readily to carbon dioxide, water and ammonia. Production Ammonium bicarbonate is produced by combining carbon dioxide and ammonia: :CO2 + NH3 + H2O → (NH4)HCO3 Since ammonium bicarbonate is thermally unstable, the reaction solution is kept cold, which allows the precipitation of the product as white solid. About 100,000 tons were produced in this way in 1997. Ammonia gas passed into a strong aqueous solution of the sesquicarbonate (a 2:1:1 mixture of (NH4)HCO3, (NH4)2CO3, and H2O) converts it into normal ammonium carbonate ((NH4)2CO3), which can be obtained in the crystalline condition from a solution prepared at about 30 °C. This compound on exposure to air gives off ammonia and reverts to ammonium bicarbonate. Salt of hartshorn Compositions co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bicarbonates
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula . Bicarbonate serves a crucial biochemical role in the physiological pH buffering system. The term "bicarbonate" was coined in 1814 by the English chemist William Hyde Wollaston. The name lives on as a trivial name. Chemical properties The bicarbonate ion (hydrogencarbonate ion) is an anion with the empirical formula and a molecular mass of 61.01 daltons; it consists of one central carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement, with a hydrogen atom attached to one of the oxygens. It is isoelectronic with nitric acid . The bicarbonate ion carries a negative one formal charge and is an amphiprotic species which has both acidic and basic properties. It is both the conjugate base of carbonic acid ; and the conjugate acid of , th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hyperkalemia
Hyperkalemia is an elevated level of potassium (K+) in the blood. Normal potassium levels are between 3.5 and 5.0mmol/L (3.5 and 5.0mEq/L) with levels above 5.5mmol/L defined as hyperkalemia. Typically hyperkalemia does not cause symptoms. Occasionally when severe it can cause palpitations, muscle pain, muscle weakness, or numbness. Hyperkalemia can cause an abnormal heart rhythm which can result in cardiac arrest and death. Common causes of hyperkalemia include kidney failure, hypoaldosteronism, and rhabdomyolysis. A number of medications can also cause high blood potassium including spironolactone, NSAIDs, and angiotensin converting enzyme inhibitors. The severity is divided into mild (5.5–5.9mmol/L), moderate (6.0–6.4mmol/L), and severe (>6.5mmol/L). High levels can be detected on an electrocardiogram (ECG). Pseudohyperkalemia, due to breakdown of cells during or after taking the blood sample, should be ruled out. Initial treatment in those with ECG changes is salts ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonation
Carbonation is the chemical reaction of carbon dioxide to give carbonates, bicarbonates, and carbonic acid. In chemistry, the term is sometimes used in place of carboxylation, which refers to the formation of carboxylic acids. In inorganic chemistry and geology, carbonation is common. Metal hydroxides (MOH) and metal oxides (M'O) react with CO2 to give bicarbonates and carbonates: :MOH + CO2 → M(HCO3) :M'O + CO2 → M'CO3 In reinforced concrete, the chemical reaction between carbon dioxide in the air and calcium hydroxide and hydrated calcium silicate in the concrete is known as neutralisation. The similar reaction in which calcium hydroxide from cement reacts with carbon dioxide and forms insoluble calcium carbonate is carbonatation. Henry's law Henry's law states that P=KBx where P is the partial pressure of gas above the solution. KB is Henry's law constant. KB increases as temperature increases. x is the mole fraction In chemistry, the mole fraction or mol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Limestone
Limestone ( calcium carbonate ) is a type of carbonate sedimentary rock which is the main source of the material lime. It is composed mostly of the minerals calcite and aragonite, which are different crystal forms of . Limestone forms when these minerals precipitate out of water containing dissolved calcium. This can take place through both biological and nonbiological processes, though biological processes, such as the accumulation of corals and shells in the sea, have likely been more important for the last 540 million years. Limestone often contains fossils which provide scientists with information on ancient environments and on the evolution of life. About 20% to 25% of sedimentary rock is carbonate rock, and most of this is limestone. The remaining carbonate rock is mostly dolomite, a closely related rock, which contains a high percentage of the mineral dolomite, . ''Magnesian limestone'' is an obsolete and poorly-defined term used variously for dolomite, for limes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caves
A cave or cavern is a natural void in the ground, specifically a space large enough for a human to enter. Caves often form by the weathering of rock and often extend deep underground. The word ''cave'' can refer to smaller openings such as sea caves, rock shelters, and grottos, that extend a relatively short distance into the rock and they are called ''exogene'' caves. Caves which extend further underground than the opening is wide are called ''endogene'' caves. Speleology is the science of exploration and study of all aspects of caves and the cave environment. Visiting or exploring caves for recreation may be called ''caving'', ''potholing'', or ''spelunking''. Formation types The formation and development of caves is known as ''speleogenesis''; it can occur over the course of millions of years. Caves can range widely in size, and are formed by various geological processes. These may involve a combination of chemical processes, erosion by water, tectonic forces, microorgani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
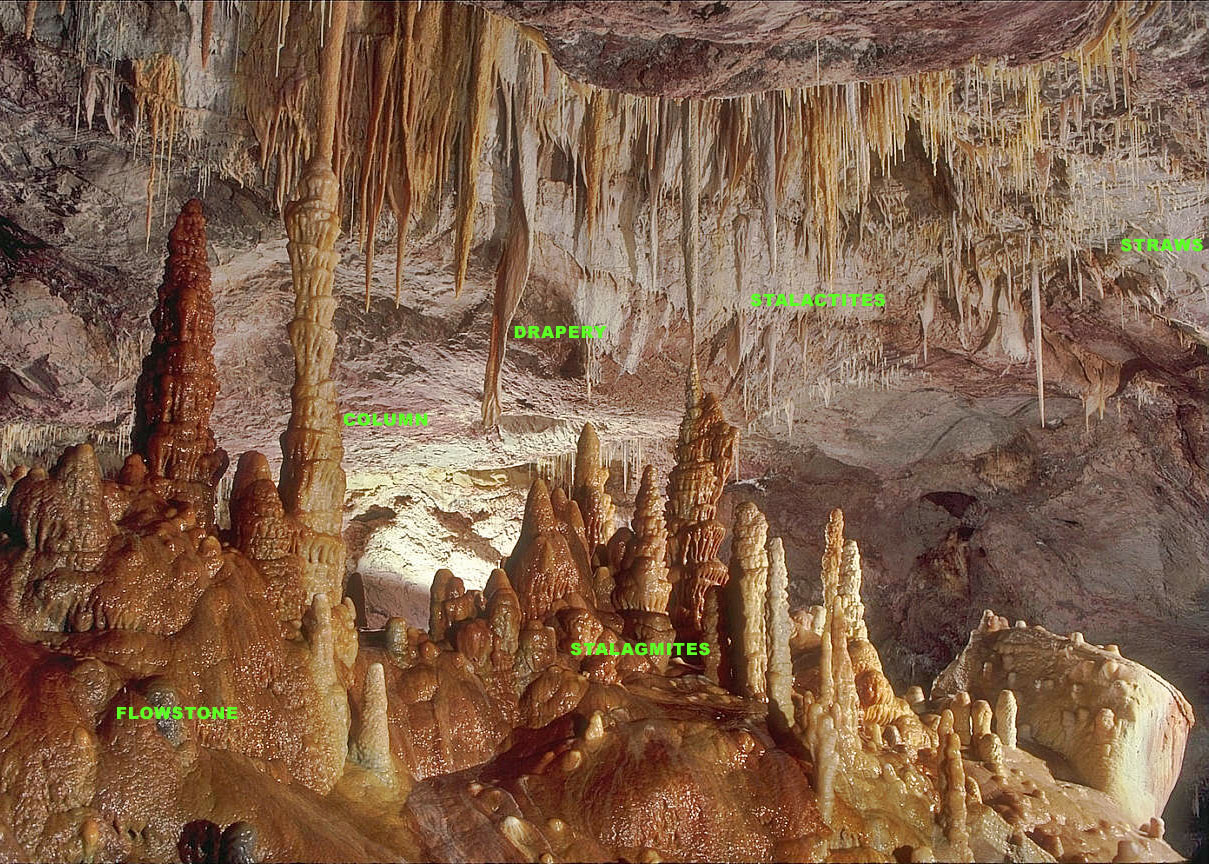
Speleothems
A speleothem (; ) is a geological formation by mineral deposition (geology), deposits that accumulate over time in natural caves. Speleothems most commonly form in calcareous caves due to carbonate dissolution reactions. They can take a variety of forms, depending on their depositional history and environment. Their chemical composition, gradual growth, and preservation in caves make them useful paleoclimatology, paleoclimatic proxies. Chemical and physical characteristics More than 300 variations of cave mineral deposits have been identified. The vast majority of speleothems are calcareous, composed of calcium carbonate (CaCO3) minerals (calcite or aragonite). Less commonly, speleothems are made of calcium sulfate (gypsum or mirabilite) or opal. Speleothems of pure calcium carbonate or calcium sulfate are translucent and colorless. The presence of iron oxide or copper provides a reddish brown color. The presence of manganese oxide can create darker colors such as black or dark b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stalagmite
A stalagmite (, ; from the Greek , from , "dropping, trickling") is a type of rock formation that rises from the floor of a cave due to the accumulation of material deposited on the floor from ceiling drippings. Stalagmites are typically composed of calcium carbonate, but may consist of lava, mud, peat, pitch, sand, sinter, and amberat (crystallized urine of pack rats). The corresponding formation hanging down from the ceiling of a cave is a stalactite. Mnemonics have been developed for which word refers to which type of formation; one is that ''stalactite'' has a C for "ceiling", and ''stalagmite'' has a G for "ground", another is that, as with ants in the pants, the mites go up and the tights (tites) come down. Formation and type Limestone stalagmites The most common stalagmites are speleothems, which usually form in limestone caves. Stalagmite formation occurs only under certain pH conditions within the cavern. They form through deposition of calcium carbonate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stalactites
A stalactite (, ; from the Greek 'stalaktos' ('dripping') via ''stalassein'' ('to drip') is a mineral formation that hangs from the ceiling of caves, hot springs, or man-made structures such as bridges and mines. Any material that is soluble and that can be deposited as a colloid, or is in suspension, or is capable of being melted, may form a stalactite. Stalactites may be composed of lava, minerals, mud, peat, pitch, sand, sinter, and amberat (crystallized urine of pack rats). A stalactite is not necessarily a speleothem, though speleothems are the most common form of stalactite because of the abundance of limestone caves. The corresponding formation on the floor of the cave is known as a stalagmite. Mnemonics have been developed for which word refers to which type of formation; one is that ''stalactite'' has a C for "ceiling", and ''stalagmite'' has a G for "ground". Another example is that ''stalactites'' "hang on ''T''ight" and ''stalagmites'' "''M''ight grow up" � ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkali Metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names for the elements in some languages, such as German and Russian. rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute Group (periodic table)#Group names, group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an atomic orbital, s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of periodic trends, group trends in properties in the periodic table, with elements exhibiting well-characterised homology (chemistry), homologous behaviour. This family of elements is also known as the lithium family after its leading element. The alkali metals are all sh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aqueous
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, or sodium chloride (NaCl), in water would be represented as . The word ''aqueous'' (which comes from ''aqua'') means pertaining to, related to, similar to, or dissolved in, water. As water is an excellent solvent and is also naturally abundant, it is a ubiquitous solvent in chemistry. Since water is frequently used as the solvent in experiments, the word solution refers to an aqueous solution, unless the solvent is specified. A ''non-aqueous solution'' is a solution in which the solvent is a liquid, but is not water. (See also Solvent and Inorganic nonaqueous solvent.) Characteristics Substances that are ''hydrophobic'' ('water-fearing') do not dissolve well in water, whereas those that are ''hydrophilic'' ('water-friendly') do. An example of a hydrophilic substance is sodium chlori ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |