|
Caesium Ozonide
Caesium ozonide (CsO3) is an oxygen-rich compound of caesium. It is an ozonide, meaning it contains the ozonide anion (O3−). It can be formed by reacting ozone with caesium superoxide: :CsO2 + O3 -> CsO3 + O2 The compound will react strongly with any water in the air forming caesium hydroxide. :2 CsO3 + H2O -> 2CsOH + 5/2O2 If heated to between 70 and 100 °C, caesium ozonide will quickly decompose to caesium superoxide (CsO2). In fact, the compound is metastable to decomposition into caesium superoxide, slowly decomposing at room temperature, but can remain intact for months if stored at -20 °C. Above around 8 °C, the crystal structure is of the caesium chloride type, with the ozonide in place of the chloride ion. At lower temperatures, the crystal structure changes to a structure identical to rubidium ozonide (RbO3), with space group In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
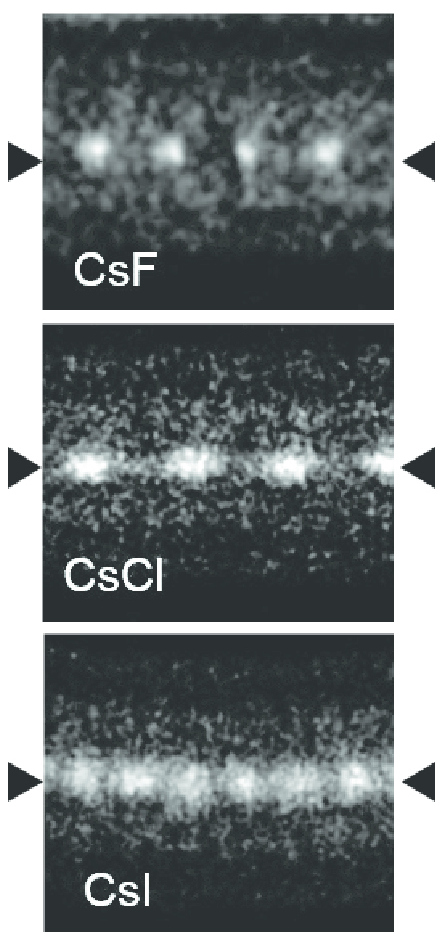
Caesium Fluoride
Caesium fluoride or cesium fluoride is an inorganic compound with the formula CsF and it is a hygroscopic white salt. Caesium fluoride can be used in organic synthesis as a source of the fluoride anion. Caesium also has the highest electropositivity of all non-radioactive elements and fluorine has the highest electronegativity of all known elements. Synthesis and properties Caesium fluoride can be prepared by the reaction of caesium hydroxide (CsOH) with hydrofluoric acid (HF) and the resulting salt can then be purified by recrystallization. The reaction is shown below: :CsOH + HF → CsF + H2O Using the same reaction, another way to create caesium fluoride is to treat caesium carbonate (Cs2CO3) with hydrofluoric acid and again, the resulting salt can then be purified by recrystallization. The reaction is shown below: :Cs2CO3 + 2 HF → 2 CsF + H2O + CO2 CsF is more soluble than sodium fluoride or potassium fluoride in organic solvents. It is available in its anhydrous for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caesium
Caesium (IUPAC spelling) (or cesium in American English) is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metals that are liquid at or near room temperature. Caesium has physical and chemical properties similar to those of rubidium and potassium. It is pyrophoric and reacts with water even at . It is the least electronegative element, with a value of 0.79 on the Pauling scale. It has only one stable isotope, caesium-133. Caesium is mined mostly from pollucite. The element has 40 known isotopes, making it, along with barium and mercury, one of the elements with the most isotopes. Caesium-137, a fission product, is extracted from waste produced by nuclear reactors. The German chemist Robert Bunsen and physicist Gustav Kirchhoff discovered caesium in 1860 by the newly developed method of flame spectroscopy. The first small-scale applications for caesium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Space Group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it unchanged. In three dimensions, space groups are classified into 219 distinct types, or 230 types if chiral copies are considered distinct. Space groups are discrete cocompact groups of isometries of an oriented Euclidean space in any number of dimensions. In dimensions other than 3, they are sometimes called Bieberbach groups. In crystallography, space groups are also called the crystallographic or Fedorov groups, and represent a description of the symmetry of the crystal. A definitive source regarding 3-dimensional space groups is the ''International Tables for Crystallography'' . History Space groups in 2 dimensions are the 17 wallpaper groups which have been known for several centuries, though the proof that the list was complete was only ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rubidium Ozonide
Rubidium ozonide is an oxygen rich compound of rubidium. It is an ozonide, meaning it contains the ozonide anion (O3−). It can be created by reacting rubidium superoxide (RbO2) with ozone (O3) in a liquid ammonia solution. :RbO2 + O3 -> RbO3 + O2 The chemical forms in two crystal structures, the low temperature α-RbO3 (P21), and β-RbO3 (P21/c) Detailed structural analysis finds the ozonide anions are significantly off-center from the surrounding rubidium atoms. Since ozonide anion is magnetic, electron paramagnetic resonance Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a method for studying materials that have unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but the spin ... measurements of rubidium ozonide have determined the g-values of the ozonide anion. References Rubidium compounds Ozonides {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caesium Chloride
Caesium chloride or cesium chloride is the inorganic compound with the formula Cs Cl. This colorless salt is an important source of caesium ions in a variety of niche applications. Its crystal structure forms a major structural type where each caesium ion is coordinated by 8 chloride ions. Caesium chloride dissolves in water. CsCl changes to NaCl structure on heating. Caesium chloride occurs naturally as impurities in carnallite (up to 0.002%), sylvite and kainite. Less than 20 tonnes of CsCl is produced annually worldwide, mostly from a caesium-bearing mineral pollucite. Caesium chloride is widely used medicine structure in isopycnic centrifugation for separating various types of DNA. It is a reagent in analytical chemistry, where it is used to identify ions by the color and morphology of the precipitate. When enriched in radioisotopes, such as 137CsCl or 131CsCl, caesium chloride is used in nuclear medicine applications such as treatment of cancer and diagnosis of myocardial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caesium Hydroxide
Caesium hydroxide is a strong base (pKa= 15.76) containing the highly reactive alkali metal caesium, much like the other alkali metal hydroxides such as sodium hydroxide and potassium hydroxide. Caesium hydroxide is corrosive enough to quickly dissolve through glass. Due to its high reactivity, caesium hydroxide is extremely hygroscopic. Laboratory caesium hydroxide is typically a hydrate. It is an anisotropic etchant of silicon, exposing octahedral planes. This technique can form pyramids and regularly shaped etch pits for uses such as Microelectromechanical systems. It is known to have a higher selectivity to etch highly p-doped silicon than the more commonly used potassium hydroxide. This compound is not commonly used in experiments due to the high extraction cost of caesium and its reactive behaviour. It acts in similar fashion to the compounds rubidium hydroxide and potassium hydroxide Potassium hydroxide is an inorganic compound with the formula K OH, and is com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caesium Superoxide
Caesium superoxide is the superoxide of caesium. It is an orange solid. Preparation Burning caesium in excess oxygen will produce caesium superoxide. : Properties Caesium superoxide's crystal structure is same as calcium carbide. It contains direct oxygen-oxygen bonding. It reacts with water to form hydrogen peroxide and caesium hydroxide. : The standard enthalpy of formation ΔHf0 of caesium superoxide is −295 kJ/mol. Caesium superoxide reacts with ozone to form caesium ozonide Caesium ozonide (CsO3) is an oxygen-rich compound of caesium. It is an ozonide, meaning it contains the ozonide anion (O3−). It can be formed by reacting ozone with caesium superoxide: :CsO2 + O3 -> CsO3 + O2 The compound will react strongly .... : References Caesium compounds Superoxides {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lower atmosphere to (dioxygen). Ozone is formed from dioxygen by the action of ultraviolet (UV) light and electrical discharges within the Earth's atmosphere. It is present in very low concentrations throughout the latter, with its highest concentration high in the ozone layer of the stratosphere, which absorbs most of the Sun's ultraviolet (UV) radiation. Ozone's odour is reminiscent of chlorine, and detectable by many people at concentrations of as little as in air. Ozone's O3 structure was determined in 1865. The molecule was later proven to have a bent structure and to be weakly diamagnetic. In standard conditions, ozone is a pale blue gas that condenses at cryogenic temperatures to a dark blue liquid and finally a violet-black soli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ozonide
Ozonide is the polyatomic anion . Cyclic organic compounds formed by the addition of ozone () to an alkene are also called ozonides. Ionic ozonides Inorganic ozonides are dark red salts. The anion has the bent shape of the ozone molecule. Inorganic ozonides are formed by burning potassium, rubidium, or caesium in ozone, or by treating the alkali metal hydroxide with ozone; this yields potassium ozonide, rubidium ozonide, and caesium ozonide respectively. They are very sensitive explosives that have to be handled at low temperatures in an atmosphere consisting of an inert gas. Lithium and sodium ozonide are extremely labile and must be prepared by low-temperature ion exchange starting from . Sodium ozonide, , which is prone to decomposition into NaOH and , was previously thought to be impossible to obtain in pure form. However, with the help of cryptands and methylamine, pure sodium ozonide may be obtained as red crystals isostructural to . Ionic ozonides are being investigate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 (called a passivation layer) that protects the foil from further corrosion.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. . Stoichiometry (the measurable relationship between reactants and chemical equations of a equation or reaction) Oxides are extraordinarily diverse in terms of stoichiometries and in terms of the structures of each stoichiometry. Most elements form oxides of more than one stoichiometry. A well known example is carbon monoxide and carbon dioxide.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caesium Superoxide
Caesium superoxide is the superoxide of caesium. It is an orange solid. Preparation Burning caesium in excess oxygen will produce caesium superoxide. : Properties Caesium superoxide's crystal structure is same as calcium carbide. It contains direct oxygen-oxygen bonding. It reacts with water to form hydrogen peroxide and caesium hydroxide. : The standard enthalpy of formation ΔHf0 of caesium superoxide is −295 kJ/mol. Caesium superoxide reacts with ozone to form caesium ozonide Caesium ozonide (CsO3) is an oxygen-rich compound of caesium. It is an ozonide, meaning it contains the ozonide anion (O3−). It can be formed by reacting ozone with caesium superoxide: :CsO2 + O3 -> CsO3 + O2 The compound will react strongly .... : References Caesium compounds Superoxides {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caesium Chloride
Caesium chloride or cesium chloride is the inorganic compound with the formula Cs Cl. This colorless salt is an important source of caesium ions in a variety of niche applications. Its crystal structure forms a major structural type where each caesium ion is coordinated by 8 chloride ions. Caesium chloride dissolves in water. CsCl changes to NaCl structure on heating. Caesium chloride occurs naturally as impurities in carnallite (up to 0.002%), sylvite and kainite. Less than 20 tonnes of CsCl is produced annually worldwide, mostly from a caesium-bearing mineral pollucite. Caesium chloride is widely used medicine structure in isopycnic centrifugation for separating various types of DNA. It is a reagent in analytical chemistry, where it is used to identify ions by the color and morphology of the precipitate. When enriched in radioisotopes, such as 137CsCl or 131CsCl, caesium chloride is used in nuclear medicine applications such as treatment of cancer and diagnosis of myocardial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |